
The role of concurrent amplification of PD-L1, PD-L2 and JAK2 in metastatic lung adenocarcinoma as a biomarker of immune checkpoint inhibitor response: a case report
Highlight box
Key findings
• This is a case of metastatic NSCLC demonstrating an exceptional response to nivolumab.
• Genetic testing revealed amplification of the PD-L1/PD-L2/JAK2 gene cluster.
What is known and what is new?
• There is still need for better markers of response to immune checkpoint inhibitors.
• Amplification of the PD-L1/PD-L2/JAK2 gene cluster may be a strong marker of anti-PD-1 therapy response.
What is the implication, and what should change now?
• This case questions whether routine genetic testing for PD-L1/PD-L2/JAK2 amplification could optimize the choice of upfront systemic therapy in NSCLC.
Introduction
Lung cancer is the second most common cancer and the leading cause of cancer mortality worldwide (1). Approximately 80-85% of lung cancer cases are classified as non-small cell lung cancer (NSCLC) with adenocarcinoma being the most common histology type, representing around 50% of all lung cancer cases (2). Traditionally, the mainstay of systemic treatment for advanced NSCLC has been platinum-based chemotherapy. In recent years, immune checkpoint inhibitors (ICI) and tyrosine kinase inhibitors (TKIs) have transformed therapy. ICIs, specifically those targeting the PD-1/PD-L1 axis, are now used routinely in patients with advanced NSCLC.
Despite these advances, most patients treated with ICIs do not respond. In patients with heightened expression of PD-L1 (≥50% of tumor cells), a marker of favorable ICI response in NSCLC, only 45% responded to Pembrolizumab as first line therapy (3). Likewise, nivolumab demonstrates a 20% response rate in the second line setting (4). These findings, in combination with the risk of developing immune related adverse events (IrAE) and the substantial cost of these agents, have fueled the search for biomarkers that predict response to ICIs. In addition to PD-L1 immunohistochemical staining, total mutation burden (TMB), mismatch repair system deficiency (dMMR) and tumor-infiltrating lymphocytes have all been associated with an increased likelihood of benefit from ICIs (5). PD-L1 expression is the only marker used routinely in NSCLC with proven predictive value. That said, many patients with PD-L1 negative tumors experience durable responses, signaling that continued investigation of predictive biomarkers is needed.
PD-L1 amplification is associated with sensitivity to ICIs in NSCLC (6). PD-L1 and PD-L2 are ligands that bind PD-1 on activated T-cells and signal for down regulation of immune activity. Tumor cells can exploit this interaction by over-expressing PD-L1 on their cell surface (7). Amplification of PD-L1 is rare in solid tumors, having been reported in only 0.7% of cases tested (8). It is commonly linked with co-amplification of PD-L2 and JAK2, as all three genes are coded for in close genomic proximity on chromosome 9p24.1 (9). Currently, testing for PD-L1 amplification is not performed routinely in clinical practice.
In this report, we present a case of lung adenocarcinoma, with RET translocation who demonstrated a robust response to nivolumab after upfront treatment with 3 cycles of cisplatin and pemetrexed. This patient was found to harbour amplification of PD-L1, PD-L2 and JAK2 on genetic testing. We present the following case in accordance with the CARE reporting checklist (available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-39/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
A Caucasian 47-year-old female with no past medical history or smoking history presented to her family physician in May 2015 for a prolonged dry cough with hemoptysis and non-pleuritic chest pain starting 4 months ago. No history of occupational exposures to asbestos. Her family history was notable for breast cancer in her mother at age 75. She underwent a chest X-ray that was markedly abnormal and proceeded to have a computed tomography (CT) chest, which showed a large left lower lung cavitary mass with thrombosis within the left inferior pulmonary vein. Bronchoscopy was performed and a biopsy was taken, which showed invasive adenocarcinoma that stained positive for TTF-1. Testing for EGFR and ALK mutations was negative. Her only medication at the time of medical oncology consultation was low-molecular weight heparin (LMWH), which was prescribed for the left inferior pulmonary vein thrombosis. Her Eastern Cooperative Oncology Group (ECOG) performance status was 1. Her staging positron emission tomography (PET) scan showed metastases to the right proximal femur, right adrenal gland, enlarged supraclavicular and paratracheal nodes, as well as multiple mesenteric lesions. Magnetic resonance imaging (MRI) brain revealed a 9 mm lesion in right temporal lobe, see Figure 1. She was started on cisplatin/ pemetrexed at standard dosing, in June 2015. She also completed radiation to right femur comprising 20 Gy in 5 fractions.
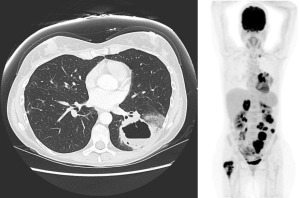
In July 2015, the patient completed her third cycle of chemotherapy, but experienced significant gastrointestinal toxicities of non-bloody diarrhea, nausea/vomiting and abdominal discomfort. Restaging CT scans demonstrated a mixed response: improvement of her supraclavicular/mediastinal adenopathy, no change to the primary tumor’s size but it was now fluid-filled and showed invasion of the left pleura. Her abdominal metastases and adrenal metastases enlarged. She started treatment with nivolumab through a compassionate access program.
The patient was admitted to hospital in September 2015 for a perforation of a small bowel metastases requiring exploratory laparotomy and small bowel resection with primary anastomosis. An intra-abdominal abscess was also drained. This resulted in a 5-week delay between her first and second dose of nivolumab. She received genetic testing by Foundation Medicine that revealed a PD-L1, PD-L2 and JAK2 amplification. The RET-KIF5B fusion was also identified. The full results are available in Table 1.
Table 1
| Gene | Type of mutation | Copy number change | FDA approved therapies# |
|---|---|---|---|
| CCND1 | Amplification | 9 | Palbociclib |
| CD274 (PD-L1) | Amplification | 8 | Nivolumab, pembrolizumab |
| JAK2 | Amplification | 8 | Ruxolitinib |
| PDCD1LG2 (PD-L2) | Amplification | 8 | Nivolumab, pembrolizumab |
| RET | KIF5B-RET fusion | N/A | Carbozantinib, lenvatinib, ponatinib, regorafenib, sorafenib, sunitinib, vandetanib |
| CDKN2A/B | Loss | N/A | None |
| FGF19 | Amplification | 9 | None |
| FGF4 | Amplification | 9 | None |
| MYC | Amplification | 21 | None |
| RICTOR | Amplification—equivocal | 7 | None |
| TP53 | R213# | N/A | None |
| FGF3 | Amplification | 9 | None |
| NKX2-1 | Amplification—equivocal | 7 | None |
#, FDA approved therapies for any tumor type at the time of genetic testing [2015]. FDA, Federal Drug Agency.
The patient continued nivolumab and experienced an excellent response at all disease sites, including her primary site, see Figure 2. In February 2016, a single enlarging lymph node was observed. Endobronchial ultrasound (EBUS) biopsy revealed a noncaseating granuloma in keeping with sarcoid reaction.
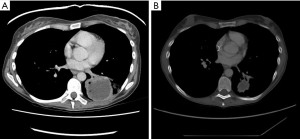
The patient’s experienced two short breaks from nivolumab occurred between January–March 2016 (cycle 11) and May–July 2016 (cycle 18) due to prolonged grade 1–2 diarrhea. Treatment resumed thereafter and the patient remained in good health aside from a partial small bowel obstruction from adhesions released surgically, keratoconjunctivitis sicca managed with artificial tears and oligo-inflammatory arthritis beginning in the left knee. The latter two symptoms were felt to represent IrAEs. In September 2017 (cycle 41), her diarrhea progressed to grade 3 level toxicity. At that point, her nivolumab was held indefinitely and she began a prednisone taper starting from 45 mg and tapering over 9 weeks. She was followed by rheumatology and prescribed methotrexate as maintenance therapy for her oligoarthritis. Serial restaging imaging was performed every 3–4 months.
In December 2020, after 43 months off treatment, the patient developed isolated mediastinal lymph node enlargement, which was in keeping with recurrent metastatic adenocarcinoma on EBUS biopsy. The patient was restarted on nivolumab March 2021. Her latest CT scans from May 2022 reveal stable mediastinal lymph nodes and multiple small nodular lung lesions with changes in keeping with inflammation rather than malignancy. An X-ray performed in June 2022 showed a new pleural effusion. The patient started a course of steroids for pleuritis, related to ICI therapy and nivolumab has been held. There is no evidence of disease progression at other sites.
Patient perspective
I am grateful for the opportunity to contribute to this case study on my treatment and response as a Stage 4 NSCLC patient. I am also grateful to my medical team for their outstanding support and care. Treatment options for lung cancer remain extremely limited although it is the most commonly diagnosed and deadliest form of cancer in North America. My response to nivolumab has been extraordinary, particularly given that we had limited information to guide decision-making as well as limited treatment options in 2015. Yet 7 years later, I am well and have more treatments options available than when I was diagnosed (Figures S1,S2).
Next-generation sequencing has been critical to developing a better understanding of my cancer and its response to nivolumab, as well as to identifying the RET-K1FB fusion in my tumour cells. I strongly believe that all cancer patients should have access to full panel biomarker testing so that they can access the best available treatments tailored to their specific form of cancer.
Knowledge that I have PD-L1 amplification and the RET fusion alteration have been a source of needed optimism and hope for me and my family as we have lived through this challenging journey. I hope that my case study will contribute to research that will generate more viable treatment options for lung and other cancer patients. I also hope that it will provide needed optimism and hope for patients and their families as we move closer to finding a cure.
Discussion
The search for biomarkers that predict response to ICIs in NSCLC is an active area of investigation. In this case of metastatic lung adenocarcinoma in a non-smoking patient, we identified amplification of PD-L1, PD-L2 and JAK2 that was associated with a long-lasting response to nivolumab.
The PD-L1 gene is coded for on chromosome 9p24.1. This chromosomal segment also encodes PD-L2 and JAK2. Amplification of this gene cluster has been identified in several malignancies, including triple negative breast cancer, diffuse large B-cell lymphoma, Hodgkin’s lymphoma, and urothelial cancer (10-12). The presence of PD-L1 amplification is associated with poorer prognoses in certain cancers, but also an increased sensitivity to ICIs (9). This was demonstrated in a recent trial of Hodgkin’s lymphoma patients failing first-line therapy with chromosome 9.24.1 amplification that showed an 86% overall response rate with nivolumab (13). It is proposed that PD-L1 amplified tumors display heightened immunotherapy sensitivity through upregulation of PD-L1 expression on tumor cell surfaces. In the case of chromosome 9p24.1 amplification, elevated JAK2 expression may be synergistic in upregulating PD-L1 expression through phosphorylation of STAT3 and STAT5, resulting in enhanced PD-L1 promoter activity (14). That said, the fact that PD-L1 amplification does not always correlate with PD-L1 expression raises the question whether there is a more complex interaction between PD-L1/PD-L2/JAK2 that confers sensitivity to ICIs irrespective of PD-L1 gene expression (15). Further work is needed to clarify this.
PD-L1 amplification is a rare finding in NSCLC with an estimated prevalence of 3%. When identified, it is usually associated with squamous histology and a history of smoking. In patients with adenocarcinoma, PD-L1 amplification is only identified in 0.6% of patients (8). Despite the low prevalence of PD-L1 amplification, it is a strong marker of ICI response when present. In a series of 5 patients collected prospectively with NSCLC and PD-L1 amplification, Inoue et al. found an 80% overall response rate with nivolumab (15). One limiting factor was the median follow-up duration of 12.6 months. Our case is a unique addition to this study, as it demonstrates a prolonged response to ICIs in a patient with a high burden of metastatic disease and greater than 7 years of follow-up.
RET driver mutations are found in approximately 1–2% of NSCLC (16). The RET-KIF5B fusion results in the formation of a supercoiled KIF5B domain that can homodimerize leading to activation of RET kinase and downstream signaling to the Ras-MAPK and PI3K-Akt pathways (17). These mutations are targetable by RET kinase inhibitors, such as Selpercatinib (18). Like most NSCLC containing a driver mutation, the presence of a RET driver mutation is associated negatively with response to ICIs (19). Interestingly, this patient was never treated with a RET kinase inhibitor and had an excellent response to nivolumab. This raises the question whether amplification of chromosome 9p24.1 predicts patients who will benefit from ICIs even in the setting of a known driver mutation. This would make for an appealing biomarker, as ICIs tend to lead to more long-lasting and robust responses.
Another important consideration is the relationship between IrAEs and response to ICI therapy. Patients who experience IrAEs have a tendency for improved outcomes on treatment (20). In this case, the patient experienced grade 1–2 colitis, oligoarthritis and keratoconjunctivitis. The link between PD-L1/PD-L2/JAK2 co-amplification and adverse events represents an area of future investigation.
This report is limited in that it reports the experience from a single patient, therefore conclusions of causation are not possible. Furthermore, this patient was initially treated in 2015 when quantification of PD-L1 expression was not routinely performed. This patient already showed exceptional response to nivolumab, so testing was deferred as it was not thought to change management. There were limitations to the Foundation Medicine Assay, as fluorescence in situ hybridization (FISH) is not included in its package. As a result, genetic testing was unable to confirm whether JAK2, PD-L1 and PD-L2 amplification arose from a single amplification of the genetic region. Given the close genomic proximity of PD-L1/PD-L2/JAK2 and equivalent gene copy numbers, we felt that amplification of the entire chromosomal locus was more likely than independent mutations.
Conclusions
In conclusion, this is a case of NSCLC with amplification of PD-L1/PD-L2/JAK2 exhibiting an excellent and long-lasting response to ICIs. Although amplification of this gene cluster is rare in NSCLC adenocarcinoma, it is a meaningful and actionable finding. This raises the question whether routine genetic testing for PD-L1/PD-L2/JAK2 amplification could optimize the choice of upfront systemic therapy and ultimately improve outcomes in this patient cohort.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-39/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-39/coif). BL has served on advisory boards for AstraZeneca, Pfizer, Bayer, Novartis and Roche. The molecular oncology diagnostics laboratory, for which he serves as Medical Director, has received research and quality improvement grant supports from Amgen, AstraZeneca, Roche and EMD Serono. The above advisory boards and work supported by the grants have not dealt directly with specific issues raised in the manuscript. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Canadian Cancer Statistics Advisory Committee. Canadian Cancer Statistics 2021:1-95.
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Bai R, Lv Z, Xu D, et al. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark Res 2020;8:34. [Crossref] [PubMed]
- Inoue Y, Yoshimura K, Mori K, et al. Clinical significance of PD-L1 and PD-L2 copy number gains in non-small-cell lung cancer. Oncotarget 2016;7:32113-28. [Crossref] [PubMed]
- Boussiotis VA. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N Engl J Med 2016;375:1767-78. [Crossref] [PubMed]
- Goodman AM, Piccioni D, Kato S, et al. Prevalence of PDL1 Amplification and Preliminary Response to Immune Checkpoint Blockade in Solid Tumors. JAMA Oncol 2018;4:1237-44. [Crossref] [PubMed]
- Ikeda S, Okamoto T, Okano S, et al. PD-L1 Is Upregulated by Simultaneous Amplification of the PD-L1 and JAK2 Genes in Non-Small Cell Lung Cancer. J Thorac Oncol 2016;11:62-71. [Crossref] [PubMed]
- George S, Papanicolau-Sengos A, Lenzo FL, et al. PD-L2 amplification and durable disease stabilization in patient with urothelial carcinoma receiving pembrolizumab. Oncoimmunology 2018;7:e1460298. [Crossref] [PubMed]
- Xue X, Huang W, Qiu T, et al. DLBCL with amplification of JAK2/PD-L2 exhibits PMBCL-like CNA pattern and worse clinical outcome resembling those with MYD88 L265P mutation. BMC Cancer 2020;20:816. [Crossref] [PubMed]
- Barrett MT, Anderson KS, Lenkiewicz E, et al. Genomic amplification of 9p24.1 targeting JAK2, PD-L1, and PD-L2 is enriched in high-risk triple negative breast cancer. Oncotarget 2015;6:26483-93. [Crossref] [PubMed]
- Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015;372:311-9. [Crossref] [PubMed]
- Prestipino A, Emhardt AJ, Aumann K, et al. Oncogenic JAK2V617F causes PD-L1 expression, mediating immune escape in myeloproliferative neoplasms. Sci Transl Med 2018;10:eaam7729. [Crossref] [PubMed]
- Inoue Y, Yoshimura K, Nishimoto K, et al. Evaluation of Programmed Death Ligand 1 (PD-L1) Gene Amplification and Response to Nivolumab Monotherapy in Non-small Cell Lung Cancer. JAMA Netw Open 2020;3:e2011818. [Crossref] [PubMed]
- Wang R, Hu H, Pan Y, et al. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J Clin Oncol 2012;30:4352-9. [Crossref] [PubMed]
- Cong XF, Yang L, Chen C, et al. KIF5B-RET fusion gene and its correlation with clinicopathological and prognostic features in lung cancer: a meta-analysis. Onco Targets Ther 2019;12:4533-42. [Crossref] [PubMed]
- Drilon A, Oxnard GR, Tan DSW, et al. Efficacy of Selpercatinib in RET Fusion-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2020;383:813-24. [Crossref] [PubMed]
- Offin M, Guo R, Wu SL, et al. Immunophenotype and Response to Immunotherapy of RET-Rearranged Lung Cancers. JCO Precis Oncol 2019; [Crossref] [PubMed]
- Teraoka S, Fujimoto D, Morimoto T, et al. Early Immune-Related Adverse Events and Association with Outcome in Advanced Non-Small Cell Lung Cancer Patients Treated with Nivolumab: A Prospective Cohort Study. J Thorac Oncol 2017;12:1798-805. [Crossref] [PubMed]
Cite this article as: Phillips WJ, Awan A, Lo B, Redway A, Nicholas G. The role of concurrent amplification of PD-L1, PD-L2 and JAK2 in metastatic lung adenocarcinoma as a biomarker of immune checkpoint inhibitor response: a case report. Precis Cancer Med 2023;6:10.

