
Chinese expert consensus recommendations for management of bone health in female patients with early breast cancer (2022 edition)
Highlight box
Key recommendations
• Tumor related treatment, concomitant basic diseases or concomitant medicine are the influencing factors of CTIBL in early breast cancer patients.
• Adjuvant bisphosphonate therapy with systemic therapy may further reduce the risk of bone metastases and may help patients gain a survival benefit.
What was recommended and what is new?
• The bone-modifying agents is recommended for Postmenopausal (natural or therapy-induced) breast cancer patients or patients in/planning for treatment with AI as soon as possible.
• Zoledronic acid is recommended for both HR+ breast cancer patients and the patients with other molecular subtyping in menopausal state (natural or therapy-induced). It is recommended on 4 mg intravenous zoledronic acid once every 3-6 months for 2-5 years.
What is the implication, and what should change now?
• In the future, we need to develop more accurate and perfect tools to manage bone loss.
Breast cancer (BC) has become one of the most common malignant tumors in China and worldwide. The latest date [2020] released by the World Health Organization (WHO) International Cancer Research Agency for cancer shows that the global number of new cases of BC is as high as 2.26 million, which has surpassed lung cancer to become the malignant tumor with the highest incidence rate in the world (1). There were more than 400,000 new cases of BC in 2020 in China (1). About 80% of BC patients are early-stage (2). With early BC patients living longer and achieving prolongation in metastasis free survival, survivorship concerns, especially with regards to bone health has attracted more and more attention. Bone health management of early BC patients includes the management of bone loss [cancer treatment induced bone loss (CTIBL) and non-tumor treatment induced bone loss (non-CTIBL)] and the prevention of bone metastasis. Ovariectomy, endocrine therapy and chemotherapy may lead to CTIBL in patients with early BC, and bone loss that has occurred before anti-tumor treatment mentioned above is usually regarded as non CTIBL. The Society of Breast Cancer of China Anti-Cancer Association and the Breast Cancer Study Group Along Yangtze River organized experts from breast surgery, oncology, and other related departments across the country to establish a consensus expert group. Referring to domestic and foreign guidelines, consensus and evidence-based medical evidence, combined with clinical experience, targeted discussion, and voting to reach a consensus, the members of the expert group developed the Chinese expert consensus recommendations for management of bone health in female patients with early BC (2022 edition), which aims to provide clinical physicians with practical bone health management plans and diagnosis and treatment measures, so as to standardize the bone health management of early BC patients (Figure 1).
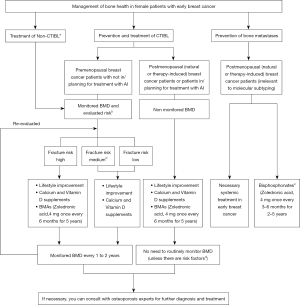
Management of CTIBL in female patients with early BC
Background
Female bone tissue begins to grow before birth and continue to increase in size, strength and density until the end of the second decade of life, by which time more than 95% of the maximum bone strength and density (peak bone mass) is acquired. After that, there will be only very slight changes in bone tissue until women reach menopause, and the rapid decline of estrogen level will lead to an increase in bone turnover and accelerate bone loss, accompanied by microstructural alterations. The overall effect of menopause is an average annual bone loss of 2–3% during the first few years and 0.5–1% thereafter. Menopause is the biggest risk factor for osteoporosis in women (3).
Recognition and diagnosis of CTIBL requires active decision making by clinicians, with notably, age and treatment of BC patients can lead to CTIBL. Given the high incidence, CTIBL is considered to be one of the most common long-term adverse reactions experienced by BC patients. Fracture caused by CTIBL has been demonstrate to significantly reduce the quality of life and survival of BC patients (4). Therefore, a routine assessment of the bone health for these patients should be performed by clinicians.
Endocrine therapy is the corner tone of adjuvant therapy for patients with hormone receptor positive (HR+) BC, but one of the key side effects of endocrine therapy due to oestrogen depletion is the occurrence of osteoporosis and fractures (5). A meta-analysis (6) suggested an increased risk of osteoporotic fractures for endocrine therapy in patients with BC. The crude risk ratio for all osteoporotic fractures was 1.35 [95% confidence interval (CI): 1.29–1.42; P<0.001], for hip fractures 1.18 (95% CI: 1.02–1.35; P<0.001), for vertebral fractures 1.84 (95% CI: 1.36–2.49; P<0.001), and for non-vertebral fractures 1.18 (95% CI: 1.02–1.35; P<0.001), respectively, compared to the controls. Fracture risk during treatment for BC may be influenced by the rate of bone mineral density (BMD) and the consequent rapid alterations in bone microarchitecture, in addition to the established fracture risk factors in postmenopausal osteoporosis (7). For post menopausal women [including those who receive ovarian function suppression (OFS) treatment before menopause], forearm, hip and spine are more prone to fracture. The mortality burden of hip fracture is significant, with a rate of approximately 3% in women aged above 50 years and hospitalized following fracture. Different from hip fractures, the risk of death after a vertebral fracture increases gradually with the extension of time after fracture. The 1-year survival rate of female patients with vertebral fracture was 86.5%, and the 5-year survival rate was 56.5%. Like hip fractures, co-morbid conditions contribute significantly towards the risk of mortality post-vertebral fracture (8). Bone loss increases the incidence of fractures, while fractures have a major impact on quality of life of BC patients. Relevant treatment for fractures will not only bring heavy mental stress to BC patients but also impose a significant economic burden to BC patients and social health system (9).
Factors and risk stratification of CTIBL
Factors
Related factors of BC treatment
The OFS treatment in premenopausal BC patients, aromatase inhibitor (AI) treatment in postmenopausal BC patients, chemotherapy, radiotherapy and ovariectomy will all significantly reduce estrogen levels and result in bone loss. Estrogen plays a leading role in the development and maintenance of women’s secondary sexual characteristics, which regulates the stability of women’s internal environment, and periodic menstruation and fertility are inseparable from the role of estrogen. Meanwhile, estrogen can inhibit osteoclasts and promote bone densification.
Estrogen has both advantages and disadvantages. The level of endogenous estrogen is significantly related to the risk of BC. The risk of BC will increase when estrogen level gradually increases. A meta-analysis analyzed the individual data from nine prospective studies showed that the BC risk associated with the doubling of estradiol levels was 1.29 (95% CI: 1.15 to 1.44; P<0.001) (10). Among early BC patients with known HR status, 70–80% BC patients were found to be HR+ (2). Estrogen and estrogen receptor (ER) combine and then activates the ER signal transduction pathway, which is the key regulatory point of tumor growth for HR+ BC patients. Endocrine therapy inhibits tumor growth by blocking the signal transduction pathway produced by estrogen or ER. A large amount of estrogen is mainly produced by ovaries in premenopausal women; therefore, OFS is the key to treatment for premenopausal HR+ BC patients. The 8-year follow-up results of SOFT study published in the New England Journal of Medicine showed that the benefit in the general population of premenopausal BC patients. The addition of OFS resulted in significantly both higher disease-free survival (DFS) [hazard ratio (HR), 0.76; 95% CI: 0.62 to 0.93; P=0.009] and overall survival (OS) (HR, 0.67; 95% CI: 0.48 to 0.92; P=0.010) than no OFS (11). Postmenopausal women mainly produce estrogen through the adrenal gland, and aromatase is the necessary key enzyme in this process. Therefore, AI is the key to the treatment of postmenopausal HR+ BC patients. Several studies (12,13) have found that AI treatment can significantly improve DFS and OS of postmenopausal BC patients.
Therefore, reducing estrogen level is an important treatment for HR+ BC patients. For premenopausal patients with early BC, OFS treatment can effectively reduce the serum estrogen level of patients, help to reach the post-menopausal state and bring survival benefits to them. For postmenopausal patients with early BC, AI treatment can significantly reduce the estrogen level and improve the survival rate of them. However, the decrease of estrogen level will lead to active osteoclasts, vigorous bone metabolism, bone loss, and seriously affect the bone health of patients (14). A study (15) found that after 2 years of OFS treatment, BMD of premenopausal patients with HR+ BC decreased by 10.5% compared with baseline. Bone loss after AI treatment was 1.6 times higher than that of normal postmenopausal women in postmenopausal patients with HR+ BC (16).
Adjuvant endocrine therapy (such as OFS and AI therapy) has become an important treatment for HR+ BC patients to reduce the risk of recurrence. The OFS treatment in premenopausal patients and AI treatment in postmenopausal patients can bring significant survival benefits to HR+ BC patients while reduce their estrogen level. Low estrogen levels will accelerate BC patients’ bone loss, affect their bone health and bring a series of bone safety problems to them. There is clear evidence-based medical evidence (17,18) that bisphosphonates [such as zoledronic acid (ZOL)] can effectively prevent or slow down bone safety problems caused by anti-tumor treatment in BC patients.
Other basic diseases
The BMD of BC patients will be affected by multiple risk factors in clinical practice. In addition to the risk factors related to tumor treatment, there are also a variety of other risk factors related to bone loss, including unhealthy lifestyle (such as lack of physical activity, smoking, alcohol abuse and excessive intake of carbonated beverages), a variety of endocrine system diseases including hypogonadism (such as premature ovarian failure), endocrine diseases (such as hyperparathyroidism, anterior pituitary hypofunction, Cushing syndrome and hyperthyroidism, etc.), rheumatic immune diseases (such as rheumatoid arthritis, ankylosing spondylitis, systemic lupus erythematosus and psoriasis, etc.), gastrointestinal diseases (such as inflammatory bowel disease, celiac disease, chronic diarrhea, pancreatic disease and primary biliary cirrhosis, etc.) Hematological diseases (such as multiple myeloma, leukemia, lymphoma, hemophilia, etc.), neuromuscular diseases (such as Parkinson’s disease, epilepsy, stroke, multiple systemic sclerosis, etc.), chronic kidney diseases (such as chronic renal insufficiency, end-stage renal disease, etc.) and cardiopulmonary diseases (such as chronic obstructive pulmonary disease, congestive heart failure, etc.) (19). In addition, some drugs can also cause bone loss, such as glucocorticoids, antacids (such as proton pump inhibitors, aluminum preparations), thiazolidinedione insulin sensitizers, anti-rejection drugs (such as tacrolimus, cyclosporin A, etc.), antidepressants, excessive thyroid hormones, anticoagulants (such as heparin), antiepileptic, anticonvulsant drugs (such as barbiturates), tumor chemotherapy drugs, selective serotonin reuptake inhibitors and antiviral drugs, etc. (20).
BMD monitor and risk classification
BMD monitor
Dual energy X-ray absorptiometry (DEXA) is a common means of diagnosis for bone loss. It is recommended to refer to the diagnostic criteria recommended by WHO for postmenopausal women, and men aged 50 and over. BMD is compared with the peak bone mass of healthy adults of the same sex and race, and it’s commonly expressed as T value. T value = (measured value − peak BMD of healthy young people of the same race and gender)/standard deviation of peak BMD of healthy young people of the same race and gender.
DEXA is the most commonly used BMD measurement method in clinical practice and scientific research. It can be used for the diagnosis of bone loss, fracture risk prediction and drug efficacy evaluation. It is also a commonly used bone health evaluation method in epidemiological research. The main measurement site is the axial bone, including the anteroposterior lumbar spine and proximal femur. If the measurement of lumbar spine and proximal femur is limited, 1/3 (33%) of the distal radius of the non-dominant side can be selected.
CTIBL risk classification and fracture risk assessment
American Society of Clinical Oncology (ASCO) and European Society for Medical Oncology (ESMO) bone health guidelines in context of early BC patients recommend that postmenopausal and elderly BC patients, post chemotherapy, endocrine therapy and post oophorectomy patients should monitor BMD routinely (19,21,22). The consensus expert group considers that BMD monitor should be routinely recommended for BC patients who have not yet menopaused and are not receiving/planning to receive AI treatment, and the risk of fracture should be assessed based on the results and clinical risk factors (Table 1) (21).
Table 1
| Risk classification | Influence factors of risk classification |
|---|---|
| Low risk | T value ≥−1.0 |
| Moderate risk | −2.0< T value <−1.0 |
| High risk | T value ≤−2.0 or −2.0< T value <−1.0 and exists any two risks at the same time (age >65 years old, T value <−1.5, smoking now and having smoking history, BMI <24 kg/m2, family history of hip fracture, personal history of fragile fracture over 50 years old, oral glucocorticoids >6 months) |
CTIBL, cancer treatment induced bone loss; BMI, body mass index.
In addition to the above recommendations, FraX®, one of the most used algorithms available online has been the common concerns of people, which was developed to estimate the 10-year risk of a major osteoporotic fracture (spine, hip, humerus or wrist) and can be used in combination with BMD assessment to identify patients with osteopenia and those with major risk of fracture (4). But FraX® was not specifically designed to assess the fracture risk of female patients receiving endocrine therapy. The fracture risk assessed by FraX® will be underestimated theoretically, but for patients with osteoporosis secondary to AI treatment, if only FraX® without BMD is used to assess the risk may overestimate the 10-year fracture incidence of patients (21). Moreover, FraX® was not validated in women under 40. In general, FraX® is not recommended to assess the fracture risk of these women.
Chinese expert consensus
- CTIBL is one of the key focus accounting clinicians, which may bring a heavy burden to patients and society (degree of consensus: 100%).
- Tumor related treatment, concomitant basic diseases or concomitant medicine are the influencing factors of CTIBL in early BC patients (degree of consensus: 75%).
- Risk classification is necessary in the management of CTIBL (degree of consensus: 100%). It is recommended to give bone-modifying agents (BMAs) if the T value is lower than a certain value (“≤−2.0” degree of consensus: 64%; “≤−2.5” degree of consensus: 25%) or −2.0< T value <−1.0 and exists any two osteoporosis risk factors at the same time (degree of consensus: 96%). Risk assessment is carried out by multidisciplinary cooperation (degree of consensus: 86%). In the future, we need to develop more accurate and perfect tools to manage bone loss (degree of consensus: 89%)
Prevention and treatment of CTIBL in female patients with early BC
Lifestyle improvement
The risk of osteoporosis has been demonstrated to increase by smoking, and also decreased after smoking cessation. In addition, alcohol consumption can affect BMD, and thus lifestyle recommendations would be smoking cessation and alcohol in moderation. The BMD is affected to some extent by drinking as well (4), so patients are recommended to stop smoking and drink properly. Exercise is widely recommended to prevent osteoporosis and can also reduce the risk of fragility fractures. Regular moderate weight-bearing exercise may benefit BMD improvement (23), but special attention should be paid to prevent falls and body impacts (23).
Calcium and vitamin D supplements
Calcium and vitamin D have been widely recommended for the prevention of osteoporosis and osteoporotic fractures. A meta-analysis showed that calcium and vitamin D supplementation can significantly reduce the risk of total fracture by up to 15% and reduce the risk of hip fracture by up to 30% (24). Calcium (1,000 mg/d) and vitamin D (800–1,000 U/d) are recommended as basic supplements. Because of endocrine therapy interfering with vitamin D absorption, higher doses of calcium and vitamin D are required (23). The International Osteoporosis Foundation recommends calcium (1,300 mg/d) and vitamin D (600 U/d) for postmenopausal women (25). The National Comprehensive Cancer Network (NCCN) recommends calcium (1,200 mg/d) and vitamin D (800–1,000 U/d) for women with high risk of CTIBL (4). The Guidelines for the diagnosis and management of primary osteoporosis of Chinese Society of Osteoporosis and Bone Mineral Research recommends calcium (1,000–1,200 mg/d) for 50 years and older and vitamin D (600 U/d) for 65 years and older (26). It also recommends 2,000 U/d is the maximum intake of vitamin D and 800–1,200 U/d is used for prevention and treatment of osteoporosis (26). Calcium supplementation and vitamin D is the basis of anti-osteoporosis drug treatment and can be used in combination with any anti-osteoporosis drug (26). The treatment response rate of bisphosphonate is effectively increased by adequate doses of vitamin D (26). Active vitamin D is more suitable for elderly patients, patients with renal dysfunction and 1α-hydroxylase deficiency or reduction, and it is good for improving BMD, reducing falls, and reducing fracture risk (26).
Use of adjuvant bisphosphonates and other BMAs
BMAs currently include bisphosphonates and denosumab. Bisphosphonates were marketed in the 1980s, and its main pharmacological effect is to inhibit osteoclast-mediated bone resorption, reduce bone loss, and improve BMD by binding to the hydroxyapatite in the bone (27,28). Different types of bisphosphonates have different anti-bone resorptive activity and bioavailability because of their large difference in chemical structures (4). Bisphosphonates has been developed to the third generation. The third-generation bisphosphonates further enhance the drug activity by extending the side chain (such as ibandronic acid and ZOL) (29).
Adjuvant endocrine therapy can effectively prevent tumor recurrence in HR+ BC patients and improve their OS after surgery (27). However, adjuvant endocrine therapy is bad for patients’ bone health because of inducing ovarian failure and then reducing estrogen levels. It may lead to CTIBL and increase fracture risk (28,30). The addition of bisphosphonates to adjuvant endocrine therapy can effectively prevent bone loss in postmenopausal (natural or therapy-induced) BC patients (Table 2).
Table 2
| Source | Type | No. of patients | Administration | Initiating BMAs | Duration (years) | BMD |
|---|---|---|---|---|---|---|
| ABCSG-12 (31,32) | Randomized, open-label, phase III, four-arm trial | 1,800; premenopausal women with hormone-responsive breast cancer | Endocrine therapy + zoledronic acid (4 mg every 6 months) vs. endocrine therapy | Starting with endocrine therapy | 3 | (I) Endocrine therapy alone caused significant BMD loss (−13.6%, mean difference −0.141 g/cm2 95% CI: 0.179 to −0.102 vs. −9.0%, mean difference −0.095 g/cm2 95% CI: −0.134 to −0.057, P<0.0001 for both) and anastrozole caused greater loss of BMD. (II) Patients who received zoledronic acid had stable BMD at 36 months (+0.4%, mean difference 0.004 g/cm2 95% CI: −0.024 to 0.032) and increased BMD at 60 months (+4.0%, mean difference 0.039 g/cm2 95% CI: 0.005–0.075, P=0.02) |
| HOBOE (18) | Multicenter, randomized, controlled, phase 3 trial | 483; HR+ patients | Premenopausal patients: OFS + TAM vs. OFS + AI vs. OFS + AI + ZOL (4 mg every 6 months); postmenopausal patients: AI vs. AI + ZOL | Starting with endocrine therapy | 5 | (I) The mean difference is between endocrine therapy + ZOL and endocrine therapy alone (+0.60, 95% CI: +0.46 to +0.77, P<0.0001). (II) the positive effect of zoledronic acid on BMD largely counteracts damage produced by letrozole as compared with tamoxifen |
| ARIBON (33,34) | Double-blind, randomized, placebo-controlled | 131; postmenopausal ER+ patients with early cancer | AI + oral ibandronate (150 mg/month) vs. AI + placebo | Starting with endocrine therapy | 5 | After 2 years, patients treated with ibandronate gained +0.60% (range, −9.0, +6.9) at the hip and +2.98% (range, −8.9, +19.9) at the lumbar spine. But Patients treated with placebo lost −3.90% (range, −12.3, +7.2) at the hip and −3.22% (range, −16.0, +4.3) at the lumbar spine. The differences between the two arms were statistically significant (P<0.01) |
| ABCSG-18 (35) | Prospective, double-blind, placebo-controlled, phase 3 trial | 3,420; postmenopausal patients with HR+ early breast cancer | AI + denosumab (60 mg every 6 months) vs. AI + placebo | Starting with endocrine therapy | 3 | (I) Patients treated with denosumab had a significantly delayed time to first clinical fracture compared with placebo (HR =0.50, 95% CI: 0.39–0.65, P<0.0001). (II) The overall lower number of fractures in the denosumab group than in the placebo group was similar in all patient subgroups, including in those with a BMD T-score <–1 already at baseline (HR =0.57, 95% CI: 0.40–0.82, P=0.002) and in patients with a BMD T-score ≥ at baseline (HR =0.44, 95% CI: 0.31–0.64, P<0.0001) |
| HALT-BC (36) | Prospective, double-blind, placebo-controlled, phase 3 trial | 252; HR+ breast cancer | AI + denosumab (60 mg every 6 months) vs. AI + placebo | Starting with endocrine therapy | 2 | The BMD increased by 5.5% and 7.6% in the denosumab group versus placebo (P<0.0001 at 12 and 24 months) |
BMD, bone mineral density; BMAs, bone-modifying agents; HR+, hormone-receptor positive; ER+, estrogen receptor positive; OFS, ovarian function suppression; TAM, tamoxifen; AI, aromatase inhibitor therapy; ZOL, zoledronic acid; HR, hazard ratio; CI, confidence interval.
Guidelines and consensus
Recommendations in ASCO Clinical Practice Guideline (19,37): BMAs may improve the BMD and reduce the risk of fracture occurrence in BC patients. ZOL is recommended for the prevention and treatment of CTIBL and denosumab is used to treat CTIBL.
Recommendations in ESMO Clinical Practice Guidelines (bone health in cancer) (21): ZOL can be used as one of the neoadjuvant and adjuvant drugs in postmenopausal (natural, OFS or ovariectomy) early BC patients [I, A]. Other patients with any one risk are recommended to receive bisphosphonate therapy [I, A] [BMD T <−2.0 or Any 2 of the following risk factors: age >65 years old, T score <−1.5, smoking (current and history of), BMI <24 kg/m2, family history of hip fracture, personal history of fragility fracture above age 50, oral glucocorticoid use for >6 months].
Recommendations in NCCN Clinical Practice Guidelines in Breast Cancer (38): BMAs have an established role as prevention and treatment of bone loss. NCCN recommendations bone health should be monitored and the use of bisphosphonates is the preferred intervention to improve BMD in BC patients treated with AI or OFS. And NCCN recommendations for use of bisphosphonates (e.g., ZOL) as adjuvant therapy and with endocrine therapy at the same time.
Recommendations in CACA-CBCS guidelines (22): Bisphosphonates can be used as therapeutic drugs in postmenopausal (natural or therapy-induced) patients during adjuvant endocrine therapy. The therapeutic options include ZOL (4 mg once every 6 months for 3–5 years) and oral clodronate (1,600 mg daily for 2–3 years).
Therapeutic options and timing
- Postmenopausal (natural or therapy-induced) BC patients or patients in/planning for treatment with AI: use BMAs as soon as possible (T values need not to be determined), such as 3 months of definitive surgery or within 2 months of completion of adjuvant chemotherapy. ZOL (4 mg once every 6 months for 5 years) is recommended firstly. Routine monitoring of BMD is not required unless the patients being affected by additional osteoporotic risk factors. The study, ZO-FAST, showed immediate ZOL therapy (patients receiving adjuvant endocrine therapy were randomly assigned to immediate ZOL) prevented CTIBL in postmenopausal women who were receiving adjuvant endocrine therapy (39-41). And the final 60-month results in ZO-FAST study (42) show immediate zoledronate is associated with improved DFS compared with endocrine therapy alone, reduced the risk of DFS events by 34% (HR =0.66; P=0.0375) with fewer local (0.9% versus 2.3%) and distant (5.5% versus 7.7%) recurrences versus delayed zoledronate (initiated for fracture or on-study BMD decrease). Five years is considered as the optimal treatment duration by ASCO-OH (CCO) guideline (23).
- Premenopausal BC patients with not in /planning for treatment with AI or patients with non-CTIBL: monitoring BMD and assessing fracture risk are needed. BMAs, calcium and Vitamin D supplements are recommended for high-risk patients [BMD T ≤−2.0, or −2.0< T <−1.0 and any 2 of the following risk factors: age >65 years old, T score <−1.5, smoking (current and history of), BMI <24 kg/m2, family history of hip fracture, personal history of fragility fracture above age 50, oral glucocorticoid use for >6 months]. ZOL (4 mg once every 3–6 months) is recommended firstly (23). BMAs is not recommended for medium-risk patients normally, but some experts still recommend it. And BMAs is not recommended for low-risk patients. The re-evaluated risk and monitored BMD every 1 to 2 years are recommended for patients at every risk grade. If necessary, you can consult with osteoporosis experts for further diagnosis and treatment.
Chinese expert consensus
- Lifestyle improvement and calcium and vitamin D supplements may prevent bone loss (degree of consensus: 95%).
- The BMAs is recommended for Postmenopausal (natural or therapy-induced) BC patients or patients in/planning for treatment with AI as soon as possible (degree of consensus: 79%). The diphosphonate for 5 years (degree of consensus: 57%) or 2–5 years (degree of consensus: 36%) is recommended. The BMAs is also recommended for patients with high-risk fracture (degree of consensus: 75%). Some experts still recommend the BMAs is used on patients with medium-risk fracture (degree of consensus: 43%).
Prevention of bone metastases in early BC
Background
Bone is the most common site of metastasis in BC patients, and 75% of patients with stage IV BC develop bone metastases (19). A large cohort study of 7,064 patients with early BC found that 22% patients was diagnosed bone metastases after a mean follow-up of 8.4 years. The mean OS of BC patients was only 40 months after diagnosis of bone metastases (42).
All subtypes of BC can all lead to bone metastases
The increased risk of bone metastasis is associated with younger, higher BC stage and higher grade. The menopausal status is not associated with bone metastases. Invasive lobular carcinoma is an independent risk factor for bone metastasis. There isn’t consensus that about the relationship between ER, progesterone receptor (PR) status and bone metastasis. The study (43) shows that the incidence of bone metastases in PR positive patients is low and the incidence of bone metastases in human epidermal growth factor receptor 2 (HER2) positive BC is relatively high.
Prevention of bone metastases in early BC
Necessary systemic treatment in early BC
The options of neoadjuvant and adjuvant systemic therapy for BC should be based on individualized assessment of the recurrence risk, molecular subtyping of tumor pathology and expected response to different treatment regimens. The doctors should choose targeted therapies such as chemotherapy, endocrine therapy, anti-HER2 therapy and further intensive treatment according to patients’ molecular subtyping and the risk of recurrence in order to prevent recurrence and metastasis.
Use of adjuvant bisphosphonates and other BMAs
The BMAs is considered to be effective drug in the treatment of bone metastasis and for adjuvant therapy in early BC (44-61) (Tables 3-5). The BMAs has the effect of preventing bone metastasis and the anti-tumor besides preventing bone loss when it is used to treat early BC (27,28), and ZOL can promote apoptosis and suppress proliferation in both tumor cells and osteoclasts (44-49).
Table 3
| Source | Type | No. of patients | Administration | Initiating bisphosphonate | Duration (years) | Median follow-up (months) | DFS | OS |
|---|---|---|---|---|---|---|---|---|
| ABCSG-12 (54) | Randomized, open-label, phase III | 1,800; premenopausal women with HR+ breast cancer | OFS + AI/TAM + ZOL (4 mg every 6 months) vs. OFS + AI/TAM | Starting with endocrine therapy | 3 | 94.4 | The ZOL being added to endocrine therapy strongly improved DFS versus endocrine therapy alone (88.4% vs. 85.0%) for an absolute increase of 3.4%. The ZOL plus endocrine therapy was risk reductions of DFS events by 23% versus endocrine therapy alone (HR =0.77, 95% CI: 0.60–0.99, P=0.042) | Absolute risk reductions with ZOL were 2.2% for OS versus endocrine therapy (96.7% vs. 94.5%) |
| HOBOE2 (55) | Multicenter, randomized, controlled, phase 3 trial | 1,065; HR + premenopausal patients with early breast cancer | OFS + TAM vs. OFS + AI vs. OFS + AI + ZOL (4 mg every 6 months) | Starting with endocrine therapy | 5 | 64 | At 5 years, the disease-free rate was respectively 85.4%, 93.2% and 93.3% with OFS + TAM, OFS + AI and OFS + AI + ZOL (overall P=0.008). The hazard ratio for a DFS event was 0.52 (95% CI: 0.34–0.80, P=0.003) with OFS + AI + ZOL vs. TAM and 0.70 (95% CI: 0.44–1.12; P=0.22) with OFS + AI + ZOL vs. OFS + AI | The overall survival rate was 96.9% (95% CI: 94.1–98.4) with OFS + TAM,98.4% (95% CI: 96.2–99.3) with OFS + AI and 99.7% (95% CI: 97.9–100.0) |
| AZURE (53) | Phase III, academic, multi-center, randomized | 3,360; women with breast cancer (HR+/HER2+/TNBC) | ZOL + (neo)adjuvant chemotherapy and/or endocrine therapy vs. (neo)adjuvant chemotherapy and/or endocrine therapy alone | Starting with (neo)adjuvant chemotherapy and/or endocrine therapy | 5 | 117 | (I) ZOL in postmenopausal women improved DFS and IDFS (HRDFS =0.82, 95% CI: 0.67–1.00; HRIDFS =0.78, 95% CI: 0.64–0.94). ZOL in women with a MAF FISH negative tumor improved IDFS (HRIDFS=0.75, 95% CI: 0.58–0.97), irrespective of menopause. (II) Bone metastases as a first DFS recurrence (BDFS) were reduced with ZOL (HRB-DFS =0.76, 95% CI: 0.63–0.92, P=0.005) | ZOL in women with a MAF FISH negative tumor improved OS (HROS =0.69, 95% CI: 0.50–0.94), irrespective of menopause |
| SUCCESS A (56) | Phase 3 multicenter randomized open-label | 3,754 | Either high-risk node-negative (defined as at least 1 of the following: age ≤35 years old, tumor size ≥pT2, negative hormone receptor status or histologic grade 3) or node-positive primary invasive breast cancer | 5 years of zoledronate treatment (4 mg ZOL every 3 months for 2 years, followed by 4mg ZOL every 6 months for 3 years) vs. 2 years of zoledronate treatment (4 mg ZOL every 3 months for 2 years) | After adjuvant chemotherapy with 6 cycles | 5 vs. 2 | Disease-free survival (HR =0.97; 95% CI: 0.75–1.25; P=0.81), and distant disease-free survival (HR =0.87; 95% CI: 0.65–1.18; P=0.38) did not differ significantly between the 2 treatment arms (5 vs. 2 years) | Overall survival (HR =0.98; 95% CI: 0.67–1.42; P=0.90) did not differ significantly between the 2 treatment arms (5 vs. 2 years) |
| EBCTCG/Meta-analyses (57) | 26 RCT | 18,766; women with early breast cancer | Endocrine therapy + bisphosphonate vs. endocrine therapy | Starting with endocrine therapy | 2–5 | 5–6 | (I) Bisphosphonate treatment produced borderline significant reductions in recurrence (RR =0.94, 95% CI: 0.87–1.01; 2P=0.08), distant recurrence (RR =0.92, 95% CI: 0.85–0.99; 2P=0.03), but it produced more definite reduction in bone recurrence (RR =0.83, 95% CI: 0.73–0.94; 2P=0.004). (I) Among postmenopausal women, bisphosphonate treatment produced highly significant reductions in recurrence (RR =0.86,95% CI: 0.78–0.94; 2P=0.002), bone recurrence (RR =0.72, 95% CI: 0.60–0.86; 2P=0.0002) and distant recurrence (RR =0.82, 95% CI: 0.74–0.92; 2P=0.0003) | (I) Bisphosphonate treatment produced borderline significant reductions in breast cancer mortality (RR =0.91,95% CI: 0.83–0.99; 2P=0.04). (II) Among postmenopausal women, bisphosphonate treatment produced highly significant reductions in breast cancer mortality (RR =0.82,95% CI: 0.73–0.93; 2P=0.002) |
| Meta-analyses 1 (58) | 8 RCT | 7,730; patients with HR+ early breast cancer | Endocrine therapy + bisphosphonate vs. endocrine therapy | Starting with endocrine therapy | 2–5 | – | The adjuvant zoledronic acid therapy improved BMFS (RR =0.81, 95% CI: 0.66–0.99, P=0.04), DMFS (RR =0.77, 95% CI: 0.60–1.00, P=0.05), FFRs (RR =0.75, 95% CI: 0.61–0.92, P=0.007) and 5-year DFS (RR =0.90, 95% CI: 0.81–1.00, P=0.06) | The adjuvant zoledronic acid therapy improved OS (RR =0.88, 95% CI: 0.77–1.01; P=0.06) and 5-year OS (RR =0.86, 95% CI: 0.7–0.99, P=0.03) in early breast cancer patients |
| Meta-analyses 2 (59) | 7 RCT | 3,969; patients with HR+ early breast cancer | Endocrine therapy + bisphosphonate vs. endocrine therapy | Starting with endocrine therapy | 2–5 | – | The risk of DFS in the patients with zoledronic acid treatment was improved in trend (HR =0.75, 95% CI: 0.52 to 1.08, P=0.121) | The patients with zoledronic acid treatment had significant longer OS than the patients without zoledronic acid treatment (HR =0.85, 95% CI: 0.73–1.00, P=0.047) |
DFS, disease-free survival; OS, overall survival; EBCTCG, Early Breast Cancer Trialists’ Collaborative Group; RCT, randomized controlled trial; HR, hazard ratio; IDFS, invasive DFS; RR, relative risk; CI, confidence interval; AI, aromatase inhibitor therapy; ZOL, zoledronic acid; HR+, hormone-receptor positive; HER2+, human epidermal growth factor receptor 2; TNBC, triple negative breast cancer; MAF, the v-maf avian musculoaponeurotic fibrosarcoma oncogene homolog gene; BMFS, bone metastasis-free survival; DMFS, distant metastasis-free survival; FFR, fracture-free rate.
Table 4
| Source | Type | No. of patients | Administration | Initiating denosumab | Duration (years) | Median follow-up (months) | DFS | OS |
|---|---|---|---|---|---|---|---|---|
| ABCSG-18 (60) | randomized, double-blind, placebo-controlled, phase 3 trial | 3,425; postmenopausal patients with early, hormone receptor-positive, non-metastatic adenocarcinoma of the breast | Denosumab (60 mg every 6 months) +AI vs. placebo + AI | During AI therapy | 3 | 96 | (I) DFS (73 months of follow-up) was significantly improved in the denosumab group versus the placebo group (HR =0.82, 95% CI: 0.69–0.98, Cox P=0.0260). (II) DFS (8 years of follow-up) was improved in the denosumab group versus the placebo group (HR =0.83,95% CI: 0.71–0.97, P=0.016). (III) bone metastasis-free survival (BMFS) was improved in the denosumab group versus the placebo group (HR =0.81,95% CI: 0.65–1.00, P=0.047) | – |
| D-CARE (61) | international, multicenter, randomized, controlled, phase 3 trial | 4,509; breast cancer (HR+, HER2+) | Systemic therapy + denosumab (120 mg, y every 3–4 weeks for about 6 months and then every 12 weeks) | Starting with neoadjuvant or adjuvant chemotherapy | 5 | 67 | (I) DFS was not significantly improved in the denosumab group versus the placebo group (HR =1.04, 95% CI: 0.91–1.19; P=0.57). (II) Bone metastasis-free survival was not significantly different (median not reached in either group, HR =0.97, 95% CI: 0.82–1.14; P=0.70) | – |
AI, aromatase inhibitor therapy; HR+, hormone-receptor positive; HER2+, human epidermal growth factor receptor 2; DFS, disease-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval.
Table 5
| Source | Type | No. of patients | Administration | Administration oral ibandronate | Duration (years) | Median follow-up (years) | DFS | OS |
|---|---|---|---|---|---|---|---|---|
| BOOG 2006-04 (62) | Randomized, open-label, multicenter phase III | 1,116; postmenopausal women with stage I-III ER+ breast cancer and an indication for adjuvant ET | ET + oral ibandronate vs. ET | oral ibandronate 50 mg once daily for 3 years | 5 years of ET and 3 years of oral ibandronate | 8.5 years | (I) Adjuvant ibandronate doesn’t improve DFS in postmenopausal patients with ER+ breast cancer (HR =0.97, 95% CI: 0.76–1.24, P=0.811). (II) Recurrence-free Interval is not significantly different between the ibandronate group and control group (HR =0.84, 95% CI: 0.62–1.14). (III) Bone Recurrence-free Interval is not significantly different between the ibandronate group and control group (HR =0.83, 95% CI: 0.55–1.25) | OS was not improved by adjuvant ibandronate (50 mg/d) in postmenopausal patients with ER+ breast cancer (HR =1.10, 95% CI: 0.82–1.49, P=0.517) |
DFS, disease-free survival; OS, overall survival; ER+, estrogen receptor positive; ET, endocrine therapy; HR, hazard ratio; CI, confidence interval.
(I) BMAs prevents bone metastasis
The large, randomized, phase III clinical trials showed adjunctive therapy combined with bisphosphonates significantly reduces the risk of recurrence, distant metastasis, and bone metastasis in postmenopausal (including premenopausal patients treated with OFS) BC patients (22). The study, AZURE (50-53), of 3,360 women with early BC to receive standard adjuvant systemic treatment alone (control group) or with 4 mg intravenous ZOL for five years with a median follow up of 84.2 months was found that adjuvant systemic treatment with ZOL effectively reduces the development of bone metastases, both as a first event (HR =0.78, 95% CI: 0.63–0.96; P=0.020) and at any time during follow-up (HR =0.81, 95% CI: 0.68–0.97; P=0.022). Bone metastases as a first DFS recurrence (B-DFS) were reduced with ZOL (HRB-DFS =0.76, 95% CI: 0.63–0.92, P=0.005) with 10 years follow-up of the AZURE randomized clinical trial (50-53). Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) produced a meta-analysis about adjuvant bisphosphonate treatment in early BC (57). The study on 18,766 women (9,290 in trials of 2–5 years of ZOL) showed bisphosphonate treatment produced highly significant reductions in BC mortality (RR =0.82, 95% CI: 0.73–0.93; 2P=0.002), recurrence (RR =0.86, 95% CI= 0.78–0.94; 2P=0.002), bone recurrence (RR=0.72, 95%CI= 0.60–0.86; 2P=0.0002) and distant recurrence (RR =0.82, 95% CI: 0.74–0.92; 2P=0.0003) among 11,767 postmenopausal women (57).
(II) BMAs and DFS/OS of BC
The results of several clinical studies and meta-analysis showed that bisphosphonates based on standard radiation therapy, chemotherapy and endocrine therapy can significantly reduce the risk of death in postmenopausal (including premenopausal patients treated with OFS) BC patients (22,50-61).
Guidelines and consensus
Recommendations in ASCO-OH (CCO) Guideline (63): bisphosphonate therapy should be discussed with all postmenopausal patients (natural or therapy-induced) with early BC who receive adjuvant systemic therapy and its hormone receptor status and human epidermal growth factor receptor 2 status is irrespective. The therapeutic options include ZOL (including the option of dosing 4 mg once every 3 months for 2 years or dosing 4 mg once every 6 months for 3 years), oral ibandronate (50 mg daily for 3 years) and oral clodronate (1,600 mg daily for 2–3 years). This is a consensus recommendation about initiating bisphosphonate within 3 months of surgery or within 2 months of completion of adjuvant chemotherapy. The use of adjuvant denosumab is not recommended.
Recommendations in ESMO Clinical Practice Guidelines (bone health in cancer) (21): Adjuvant bisphosphonates (e.g., ZOL) should be used for all postmenopausal patients or premenopausal patients with (Neo)adjuvant treatment includes ovarian suppression therapy or oophorectomy [I, A]. It is recommended to initiate bisphosphonate within (neo)adjuvant treatment (where indicate) for 5 years [I, A]. Denosumab is not recommended for the prevention of metastasis [I, D].
Recommendations in NCCN Clinical Practice Guidelines in Breast Cancer (38): The NCCN Panel recommends treatment with a BMAs such as ZOL in addition to chemotherapy or endocrine therapy can prevent and treat bone metastasis. A meta-analysis of data from seven bisphosphonate trials (AZURE, ZO-FAST, Z-FAST, EZO-FAST, ABCSG-12, GAIN, NSABP-B34) showed a significant benefit for the use of bisphosphonates in patients with early BC and a low-estrogen state (including postmenopausal, premenopausal with ovarian suppression or only those known to be aged 50 years or older).
Recommendations in CACA-CBCS guidelines (22): bisphosphonates may prevent bone metastasis and have potential effects against visceral metastasis. Several clinical studies of bisphosphonates in preventing bone metastases are still ongoing.
Therapeutic options and timing
This is a consensus recommendation that bisphosphonate therapy should be used for early BC with a low-estrogen state (29). ZOL (4 mg once every 3–6 months for 2–5 years) is recommended firstly. If there is a conflict between prevention and treatment of bone loss and prevention of bone metastasis, higher frequent mode of administration is preferred. The existing evidence proves that the mode of administration is safe. The incidence of medication-related osteonecrosis of the jaw (MRONJ) is low and can be prevented, and reasonable preventive measures can reduce the risk of it (29).
Chinese expert consensus
- Necessary systemic therapy is an important way to prevent bone metastasis in early-stage BC patients. Adjuvant bisphosphonate therapy with systemic therapy may further reduce the risk of bone metastases and may help patients gain a survival benefit (degree of consensus: 100%).
- At present, ZOL is found that the evidence-based medicine (EBM) of it is the best. ZOL is recommended for both HR+ BC patients (degree of consensus: 100%) and the patients with other molecular subtyping in menopausal state (natural or therapy-induced) (degree of consensus: 32%). It is recommended on 4 mg intravenous ZOL once every 3–6 months for 2–5 years (degree of consensus: 61%).
- It is safe on the present use of BMAs in early BC, but there were still rare adverse reactions, so oral health therapy (such as oral hygiene instruction, comprehensive oral examination, and tooth extraction) is recommended before tumor-related treatment (degree of consensus: 85%).
Acknowledgments
This article is a translation based on a consensus first reported in Chinese in China Oncology 2022;32(3):274-286, with permission acquired.
Funding: None.
Footnote
Peer Review File: Available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-57/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-57/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Colombet M, Soerjomataram I, et al. Cancer statistics for the year 2020: An overview. Int J Cancer 2021; Epub ahead of print. [Crossref] [PubMed]
- Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 2014;106:dju055. [Crossref] [PubMed]
- Rizzoli R, Bischoff-Ferrari H, Dawson-Hughes B, et al. Nutrition and bone health in women after the menopause. Womens Health (Lond) 2014;10:599-608. [Crossref] [PubMed]
- Diana A, Carlino F, Giunta EF, et al. Cancer Treatment-Induced Bone Loss (CTIBL): State of the Art and Proper Management in Breast Cancer Patients on Endocrine Therapy. Curr Treat Options Oncol 2021;22:45. [Crossref] [PubMed]
- Rachner TD, Coleman R, Hadji P, et al. Bone health during endocrine therapy for cancer. Lancet Diabetes Endocrinol 2018;6:901-10. [Crossref] [PubMed]
- Lee YK, Lee EG, Kim HY, et al. Osteoporotic Fractures of the Spine, Hip, and Other Locations after Adjuvant Endocrine Therapy with Aromatase Inhibitors in Breast Cancer Patients: a Meta-analysis. J Korean Med Sci 2020;35:e403. [Crossref] [PubMed]
- Body JJ. Increased fracture rate in women with breast cancer: a review of the hidden risk. BMC Cancer 2011;11:384. [Crossref] [PubMed]
- Clynes MA, Harvey NC, Curtis EM, et al. The epidemiology of osteoporosis. Br Med Bull 2020;133:105-17. [PubMed]
- Rashki Kemmak A, Rezapour A, Jahangiri R, et al. Economic burden of osteoporosis in the world: A systematic review. Med J Islam Repub Iran 2020;34:154. [Crossref] [PubMed]
- Key T, Appleby P, Barnes I, et al. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst 2002;94:606-16. [Crossref] [PubMed]
- Francis PA, Pagani O, Fleming GF, et al. Tailoring Adjuvant Endocrine Therapy for Premenopausal Breast Cancer. N Engl J Med 2018;379:122-37. [Crossref] [PubMed]
- Cuzick J, Sestak I, Baum M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol 2010;11:1135-41. [Crossref] [PubMed]
- Regan MM, Neven P, Giobbie-Hurder A, et al. Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor-positive breast cancer: the BIG 1-98 randomised clinical trial at 8·1 years median follow-up. Lancet Oncol 2011;12:1101-8. [Crossref] [PubMed]
- Nicks KM, Fowler TW, Akel NS, et al. Bone turnover across the menopause transition: The role of gonadal inhibins. Ann N Y Acad Sci 2010;1192:153-60. [Crossref] [PubMed]
- Fogelman I, Blake GM, Blamey R, et al. Bone mineral density in premenopausal women treated for node-positive early breast cancer with 2 years of goserelin or 6 months of cyclophosphamide, methotrexate and 5-fluorouracil (CMF). Osteoporos Int 2003;14:1001-6. [Crossref] [PubMed]
- Hadji P, Ziller M, Kieback DG, et al. Effects of exemestane and tamoxifen on bone health within the Tamoxifen Exemestane Adjuvant Multicentre (TEAM) trial: results of a German, 12-month, prospective, randomised substudy. Ann Oncol 2009;20:1203-9. [Crossref] [PubMed]
- Hadji P, Gnant M, Body JJ, et al. Cancer treatment-induced bone loss in premenopausal women: a need for therapeutic intervention? Cancer Treat Rev 2012;38:798-806. [Crossref] [PubMed]
- Nuzzo F, Gallo C, Lastoria S, et al. Bone effect of adjuvant tamoxifen, letrozole or letrozole plus zoledronic acid in early-stage breast cancer: the randomized phase 3 HOBOE study. Ann Oncol 2012;23:2027-33. [Crossref] [PubMed]
- Burstein HJ, Lacchetti C, Anderson H, et al. Adjuvant Endocrine Therapy for Women With Hormone Receptor-Positive Breast Cancer: ASCO Clinical Practice Guideline Focused Update. J Clin Oncol 2019;37:423-38. [Crossref] [PubMed]
- Ma YZ, Wang YP, Liu Q, et al. 2018 China guideline for the diagnosis and treatment of senile osteoporosis. Chin J Gerontol 2019;39:2557-75.
- Coleman R, Hadji P, Body JJ, et al. Bone health in cancer: ESMO Clinical Practice Guidelines. Ann Oncol 2020;31:1650-63. [Crossref] [PubMed]
- The Society of Breast Cancer China Anti-Cancer Association. Guidelines for breast cancer diagnosis and treatment by China Anti-Cancer Association (2021 edition). China Oncol 2021;31:954-1040.
- Handforth C, D'Oronzo S, Coleman R, et al. Cancer Treatment and Bone Health. Calcif Tissue Int 2018;102:251-64. [Crossref] [PubMed]
- Weaver CM, Alexander DD, Boushey CJ, et al. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int 2016;27:367-76. [Crossref] [PubMed]
- Hadji P, Aapro MS, Body JJ, et al. Management of aromatase inhibitor-associated bone loss in postmenopausal women with breast cancer: practical guidance for prevention and treatment. Ann Oncol 2011;22:2546-55. [Crossref] [PubMed]
- Chinese Society of Osteoporosis and Bone Mineral Research. Guidelines for the diagnosis and treatment of primary osteoporosis (2017). Chin Gen Pract 2017;20:3963-82.
- Shao ZM, Shen ZZ, Xu BH. Breast oncology. Shanghai: Fudan University Press; 2013.
- Mei M, Xiang Z, Yang J, et al. Efficacy of zoledronic acid for prevention of bone loss in early-stage breast cancer patients receiving adjuvant therapy: A meta-analysis of 13 randomized controlled trials. Curr Probl Cancer 2020;44:100507. [Crossref] [PubMed]
- Breast Cancer Group, Chinese Medical Doctor Association. International Medical Society, Chinese Anti-cancer Association. Expert consensus on safety management of bone-modifying agents. Zhonghua Zhong Liu Za Zhi 2021;43:622-8.
- Vehmanen LK, Elomaa I, Blomqvist CP, et al. The effect of ovarian dysfunction on bone mineral density in breast cancer patients 10 years after adjuvant chemotherapy. Acta Oncol 2014;53:75-9. [Crossref] [PubMed]
- Gnant MF, Mlineritsch B, Luschin-Ebengreuth G, et al. Zoledronic acid prevents cancer treatment-induced bone loss in premenopausal women receiving adjuvant endocrine therapy for hormone-responsive breast cancer: a report from the Austrian Breast and Colorectal Cancer Study Group. J Clin Oncol 2007;25:820-8. [Crossref] [PubMed]
- Gnant M, Mlineritsch B, Luschin-Ebengreuth G, et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 5-year follow-up of the ABCSG-12 bone-mineral density substudy. Lancet Oncol 2008;9:840-9. [Crossref] [PubMed]
- Lester JE, Dodwell D, Purohit OP, et al. Prevention of anastrozole-induced bone loss with monthly oral ibandronate during adjuvant aromatase inhibitor therapy for breast cancer. Clin Cancer Res 2008;14:6336-42. [Crossref] [PubMed]
- Lester JE, Dodwell D, Brown JE, et al. Prevention of anastrozole induced bone loss with monthly oral ibandronate: Final 5 year results from the ARIBON trial. J Bone Oncol 2012;1:57-62. [Crossref] [PubMed]
- Gnant M, Pfeiler G, Dubsky PC, et al. Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2015;386:433-43. [Crossref] [PubMed]
- Ellis GK, Bone HG, Chlebowski R, et al. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol 2008;26:4875-82. [Crossref] [PubMed]
- Shapiro CL, Van Poznak C, Lacchetti C, et al. Management of Osteoporosis in Survivors of Adult Cancers With Nonmetastatic Disease: ASCO Clinical Practice Guideline. J Clin Oncol 2019;37:2916-46. [Crossref] [PubMed]
- NCCN clinical practice guidelines in oncology (breast cancer) (version 5.2021). June 28, 2021. Available online: http://www.nccn.org/patients
- Bundred NJ, Campbell ID, Davidson N, et al. Effective inhibition of aromatase inhibitor-associated bone loss by zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: ZO-FAST Study results. Cancer 2008;112:1001-10. [Crossref] [PubMed]
- Eidtmann H, de Boer R, Bundred N, et al. Efficacy of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36-month results of the ZO-FAST Study. Ann Oncol 2010;21:2188-94. [Crossref] [PubMed]
- Coleman R, de Boer R, Eidtmann H, et al. Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): final 60-month results. Ann Oncol 2013;24:398-405. [Crossref] [PubMed]
- Harries M, Taylor A, Holmberg L, et al. Incidence of bone metastases and survival after a diagnosis of bone metastases in breast cancer patients. Cancer Epidemiol 2014;38:427-34. [Crossref] [PubMed]
- Yamashiro H, Takada M, Nakatani E, et al. Prevalence and risk factors of bone metastasis and skeletal related events in patients with primary breast cancer in Japan. Int J Clin Oncol 2014;19:852-62. [Crossref] [PubMed]
- Derenne S, Amiot M, Barillé S, et al. Zoledronate is a potent inhibitor of myeloma cell growth and secretion of IL-6 and MMP-1 by the tumoral environment. J Bone Miner Res 1999;14:2048-56. [Crossref] [PubMed]
- Aparicio A, Gardner A, Tu Y, et al. In vitro cytoreductive effects on multiple myeloma cells induced by bisphosphonates. Leukemia 1998;12:220-9. [Crossref] [PubMed]
- Fromigue O, Lagneaux L, Body JJ. Bisphosphonates induce breast cancer cell death in vitro. J Bone Miner Res 2000;15:2211-21. [Crossref] [PubMed]
- Senaratne SG, Pirianov G, Mansi JL, et al. Bisphosphonates induce apoptosis in human breast cancer cell lines. Br J Cancer 2000;82:1459-68. [Crossref] [PubMed]
- Boissier S, Ferreras M, Peyruchaud O, et al. Bisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastases. Cancer Res 2000;60:2949-54. [PubMed]
- Wood J, Bonjean K, Ruetz S, et al. Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. J Pharmacol Exp Ther 2002;302:1055-61. [Crossref] [PubMed]
- Coleman R, Woodward E, Brown J, et al. Safety of zoledronic acid and incidence of osteonecrosis of the jaw (ONJ) during adjuvant therapy in a randomised phase III trial (AZURE: BIG 01-04) for women with stage II/III breast cancer. Breast Cancer Res Treat 2011;127:429-38. [Crossref] [PubMed]
- Coleman R, Cameron D, Dodwell D, et al. Adjuvant zoledronic acid in patients with early breast cancer: final efficacy analysis of the AZURE (BIG 01/04) randomised open-label phase 3 trial. Lancet Oncol 2014;15:997-1006. [Crossref] [PubMed]
- Coleman R, Hall A, Albanell J, et al. Effect of MAF amplification on treatment outcomes with adjuvant zoledronic acid in early breast cancer: a secondary analysis of the international, open-label, randomised, controlled, phase 3 AZURE (BIG 01/04) trial. Lancet Oncol 2017;18:1543-52. [Crossref] [PubMed]
- Coleman RE, Collinson M, Gregory W, et al. Benefits and risks of adjuvant treatment with zoledronic acid in stage II/III breast cancer. 10 years follow-up of the AZURE randomized clinical trial (BIG 01/04). J Bone Oncol 2018;13:123-35. [Crossref] [PubMed]
- Gnant M, Mlineritsch B, Stoeger H, et al. Zoledronic acid combined with adjuvant endocrine therapy of tamoxifen versus anastrozol plus ovarian function suppression in premenopausal early breast cancer: final analysis of the Austrian Breast and Colorectal Cancer Study Group Trial 12. Ann Oncol 2015;26:313-20. [Crossref] [PubMed]
- Perrone F, De Laurentiis M, De Placido S, et al. Adjuvant zoledronic acid and letrozole plus ovarian function suppression in premenopausal breast cancer: HOBOE phase 3 randomised trial. Eur J Cancer 2019;118:178-86. [Crossref] [PubMed]
- Friedl TWP, Fehm T, Müller V, et al. Prognosis of Patients With Early Breast Cancer Receiving 5 Years vs 2 Years of Adjuvant Bisphosphonate Treatment. JAMA Oncol 2021;7:1149-57. [Crossref] [PubMed]
- Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet 2015;386:1353-61. [Crossref] [PubMed]
- He M, Fan W, Zhang X. Adjuvant zoledronic acid therapy for patients with early stage breast cancer: an updated systematic review and meta-analysis. J Hematol Oncol 2013;6:80. [Crossref] [PubMed]
- Huang WW, Huang C, Liu J, et al. Zoledronic acid as an adjuvant therapy in patients with breast cancer: a systematic review and meta-analysis. PLoS One 2012;7:e40783. [Crossref] [PubMed]
- Gnant M, Pfeiler G, Steger GG, et al. Adjuvant denosumab in postmenopausal patients with hormone receptor-positive breast cancer (ABCSG-18): disease-free survival results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019;20:339-51. [Crossref] [PubMed]
- Coleman R, Finkelstein DM, Barrios C, et al. Adjuvant denosumab in early breast cancer (D-CARE): an international, multicentre, randomised, controlled, phase 3 trial. Lancet Oncol 2020;21:60-72. [Crossref] [PubMed]
- Vliek SB, Noordhoek I, Meershoek-Klein Kranenbarg E, et al. Daily Oral Ibandronate With Adjuvant Endocrine Therapy in Postmenopausal Women With Estrogen Receptor-Positive Breast Cancer (BOOG 2006-04): Randomized Phase III TEAM-IIB Trial. J Clin Oncol 2022;40:2934-45. [Crossref] [PubMed]
- Eisen A, Somerfield MR, Accordino MK, et al. Use of Adjuvant Bisphosphonates and Other Bone-Modifying Agents in Breast Cancer: ASCO-OH (CCO) Guideline Update. J Clin Oncol 2022;40:787-800. [Crossref] [PubMed]
Cite this article as: The Society of Breast Cancer of China Anti-Cancer Association, Breast Cancer Study Group Along Yangtze River. Chinese expert consensus recommendations for management of bone health in female patients with early breast cancer (2022 edition). Precis Cancer Med 2022;5:33.

