
Tract injection of autologous blood (intraparenchymal blood patching) in percutaneous transthoracic CT-guided lung biopsy and the incidence of pneumothorax: a retrospective analysis
Introduction
Percutaneous transthoracic computed tomography (CT)-guided lung biopsy is a common procedure performed in the diagnostic evaluation of lung lesions. One of the most common complications of this procedure is pneumothorax. This exposes patients to the possibility of requiring a chest tube with an extended hospital stay, serial radiological imaging, and in some cases, evaluation and treatment for bronchopleural fistula. A meta-analysis of 32 studies investigating complications of lung biopsy reported pneumothorax incidence rates between 22.2% to 28.6% in patients undergoing core lung biopsy, with 4.3% to 7.3% of the patients requiring either manual aspiration or chest tube placement and/or hospitalization (1).
There have been mixed results in the literature from studies investigating the efficacy of blood patching. Bourgouin et al. (2), did find a decrease in the incidence of pneumothorax with blood patching from 34.1% to 28.8%, along with a decrease in the incidence of pneumothorax requiring chest tube placements from 9.1% to 7.7%. Similar results were demonstrated by Herman and Weisbrod (3), with a decrease in incidence of pneumothorax with blood patching from 30% to 24%, however an insignificant increase was noted in patients needing chest tube placement in the blood patching group (2.2%, 1/46 vs. 2.1%, 1/47), but the findings were not statistically significant in both studies (2,3). A randomized controlled trial published in 2013 consisting of 242 patients found reduction in the incident rate of pneumothorax from 35% to 26% (P=0.12) and significant reduction in the rate of pneumothorax requiring a chest tube from 18% to 9% (P=0.048) (4).
A recent retrospective review has showed promise for the efficacy of blood patching (5). Graffy et al. [2017] (5) found that with use of intraparenchymal blood (IPB) they had a significant reduction in pneumothorax from 44% to 30% (P<0.0001), and a reduction in pneumothorax related interventions, 24.1% to 8.9% (P<0.0001), including chest tube placement, 6.8% to 3.1% (P<0.0001), and hospital admission, 7.7% to 3.7% (P<0.0001).
Current standard of care guidelines and recommendations do not warrant the instillation of autologous blood routinely, likely due to lack of enough evidence, but does mention the benefit noted in the literature (6). The goal of this retrospective review is to compare the rates of post-biopsy pneumothorax, chest tube placement, and hospitalization when IPB is used. We present the following article in accordance with the STROBE reporting checklist (available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-9/rc).
Methods
Patient selection
This retrospective study is compliant with the Health Insurance Portability and Accountability Act and approved by the Institutional Review Board of Ascension St. John Hospital (IRB No. 1589530). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived. Patients were identified using ICD-9 and ICD-10 codes. All percutaneous CT-guided biopsies performed at two hospitals from January 1st, 2013 through January 1st, 2020 were collected summing to 848 potentially eligible studies.
Patients were required to be eighteen years-of-age or older to be included. Studies were excluded if an alternative sealant method was used, an indwelling chest tube was already in place, and/or if lung parenchyma was not traversed by the guiding needle to reach the lesion of concern. For patients who had undergone multiple biopsies, only the first study was included in statistical analysis. Studies were also excluded if procedural images were unavailable for review. There were 536 total studies included for analysis; 227 had not received blood patching, 309 had received IPB.
Biopsy technique
Out of the 536 biopsies, 528 were performed using a 19-G introducer needle with an associated 20-G core biopsy needle. A 17-G introducer needle was used on the eight other biopsies. The patients were appropriately placed on the CT table and biopsies were performed under CT guidance. All safe techniques were utilized in the biopsy. If possible, the side being biopsied was kept dependent. Traversing through the fissures was avoided unless the lesions were not otherwise accessible. Bullae, blood vessels, and airways were avoided. The time for which the needle was within the patient was kept to the minimum. If there were multiple possible tracts, the tract that required traversing through the least amount of lung tissue was selected.
Blood patching technique
The utilization of blood patching was determined by manual review of procedural records. Blood patching was not performed in patients from 2013–2015; however, all patients who underwent CT-guided lung biopsy from 2016 to 2019 received blood patching.
At the time of intravenous (IV) access, approximately 10 mL of venous blood is withdrawn from the patient into a syringe. The syringe is left undisturbed throughout the procedure to allow for the blood to clot or separate. The distance of the pleura from skin is calculated on the pre-biopsy planning scan. After the biopsy specimens are obtained and immediately before the coaxial introducer needle is removed, plasma is slowly separated from the blood/clots in the syringe and the remaining blood/clots are injected into the tract as the guiding needle is slowly removed. The injection is continued up to and just past the pleura. Typically, 4–6 mL is injected, depending on the tract length.
Pneumothorax
Patients with a large pneumothorax found immediately after biopsy on CT-imaging, even before removal of the biopsy needle, were excluded from the study. Pneumothorax size was calculated using the equation Y = 4.2 + (4.7 × (A + B + C)), r=0.98, (P<0.0001), developed by Collins et al. (7). The post-procedural CT scan images and follow-up chest radiographs were evaluated for pneumothorax. If post-biopsy pneumothorax was identified, serial chest radiographs were performed hourly for 2 hours until pneumothorax stability was confirmed, or a chest tube was placed for an enlarging pneumothorax or if the patient was becoming symptomatic. Patients with chest tubes were admitted for 24-hour observation. The chest tube was removed if there was no pneumothorax on the morning chest radiograph with clamping of the tube.
Chest tube placement
The decision for chest tube placement was determined by the patient’s symptom severity and pneumothorax chest radiograph. Patients with a symptomatic pneumothorax, enlarging pneumothorax, or pneumothorax >25% volume were treated with 8.5 Fr Cook multi-purposed chest tubes that were placed under CT guidance. The patients receiving chest tubes were admitted and were managed by either the in-house thoracic surgery team or an interventional radiologist.
Statistical analysis
Descriptive statistics were given for all data collected. Missing data were not replaced by substitutions or computations. Categorical variables were provided as counts and % frequencies. All continuous variables were provided as either means ± the standard deviation or median and 25th and 75th percentiles, followed by the minimum to maximum dependent on the normality of the data. All analyses used SAS® System for Windows version 9.4 or higher, Cary, NC.
Baseline and demographic data were compared between patients receiving autologous blood patch and those that did not receive autologous blood patch injections. Univariate analyses were done using Chi-square tests for categorical variables where appropriate (expected frequency >5 in 80% of cells), otherwise Fisher’s Exact tests were used. Continuous variables were examined using Wilcoxon rank-sum tests or t-tests dependent on the normality of the data.
The primary outcomes of post-biopsy pneumothorax, the need for chest tube placement and hospital admission were each examined separately between the patients receiving autologous blood patch injections and those that did not receive autologous blood patch injections using Chi-square tests where appropriate (expected frequency >5 in 80% of cells), otherwise Fisher’s Exact tests were used.
Results
Approximately half of the subjects were males. The average age was 69 years. The most common indication for biopsy was the characterization of a new lesion at 81.5%, while 11% was for a prior lung lesion that had changed in size. 82.8% of the total subjects reported a history of cigarette smoking. There was a higher percentage of patients who reported a history of chronic obstructive pulmonary disease (COPD) in the control group (46.7% vs. 35.4%; P<0.008), however, this was obtained from patient chart review and not by radiological evidence of emphysema. The presence of emphysema has a notable impact on the likelihood of developing a pneumothorax and a pneumothorax necessitating chest tube placement (8). Refer Table 1 for patient demographics and baseline characteristics.
Table 1
| Baseline characteristics | No blood patch (N=227) | Blood patch (N=309) | P value |
|---|---|---|---|
| Males | 107 (47.1%) | 146 (47.3%) | 0.98 |
| Age (years) | 0.25 | ||
| Mean ± SD [median] | 68±11 [69] | 69±10 [70] | |
| Min to max | 25–93 | 41–92 | |
| Smoker | 0.036 | ||
| 0= never | 28 (12.3%) | 64 (20.7%) | |
| 1= prior | 100 (44.1%) | 118 (38.2%) | |
| 2= current | 99 (43.6%) | 127 (41.1%) | |
| History of asthma | 14 (6.2%) | 21 (6.8%) | 0.78 |
| History of diabetes | 36 (15.9%) | 70 (22.7%) | 0.051 |
| History of COPD | 106 (46.7%) | 109 (35.4%) | 0.008 |
| Home oxygen required | 16 (7.1%) | 6 (1.9%) | 0.003 |
| Prior lung surgery | 8 (3.5%) | 10 (3.2%) | 0.85 |
| Prior lung radiation | 4 (1.8%) | 4 (1.3%) | 0.73 |
| Incidental findings on routine screening | 99 (43.6%) | 145 (46.9%) | 0.45 |
| Shortness of breath | 41 (18.1%) | 67 (21.7%) | 0.30 |
| Chronic cough | 27 (11.9%) | 34 (11.0%) | 0.75 |
| Chest pain | 31 (13.7%) | 35 (11.3%) | 0.42 |
| Hemoptysis | 7 (3.1%) | 8 (2.6%) | 0.73 |
| Reasons for biopsy | |||
| New lesion | 196 (86.3%) | 241 (78.0%) | 0.014 |
| Old lesion size change | 28 (12.3%) | 31 (10.0%) | 0.40 |
| Old lesion negative biopsy | 0 | 2 (0.7%) | 0.51 |
| Needle size | 0.15 | ||
| 17 gauge | 1 (0.4%) | 7 (2.3%) | |
| 19 gauge | 226 (99.6%) | 302 (97.7%) |
Data are presented as n (%) unless indicated otherwise. COPD, chronic obstructive pulmonary disease.
There was a significant decrease in incidence of pneumothorax from 37.0% to 14.6% (P<0.0001), chest-tube placement from 17.2% to 3.9% (P<0.0001), and hospital admissions from 16.3% to 2.9% (P<0.0001) in the subjects who received post-biopsy IPB compared to those who did not. Length of hospitalization did not vary significantly between the two groups. There were no reported adverse effects related to blood patching (Table 2 and Figure 1).
Table 2
| Complications | No blood patch (N=227) | Blood patch (N=309) | P value |
|---|---|---|---|
| Pneumothorax | 84 (37.0%) | 45 (14.6%) | <0.0001 |
| Chest tube placed | 39 (17.2%) | 12 (3.9%) | <0.0001 |
| Hospitalized | 37 (16.3%) | 9 (2.9%) | <0.0001 |
| Days hospitalized (N) | 37 | 9 | 0.91 |
| Median [25th, 75th] | 3 [2, 5] | 3 [2, 5] | |
| Min to max | 1–21 | 1–9 | |
| Lung biopsy result (malignant) | 162/224 (72.3%) | 207 (67.0%) | 0.19 |
Data are presented as n (%) unless indicated otherwise.
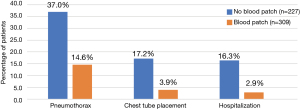
In patients who did experience pneumothorax, size of pneumothorax was significantly smaller in the group who did receive IPB (Table 3).
Table 3
| Pneumothorax size | No blood patch (n=227) | Blood patch (n=309) | P value |
|---|---|---|---|
| Median | 16% | 9% | 0.004 |
| First quartile | 8% | 7% | 0.004 |
| Third quartile | 29% | 13% | 0.004 |
| Minimum to maximum | 5–55% | 4–49% | 0.004 |
Discussion
Previously, very few studies have been performed to compare the incidence rates of complications in patients undergoing CT-guided lung biopsy. Moreover, mixed results were found in previous studies, which underscores the importance of the current study (9-14). In this retrospective analysis of 536 lung biopsies, we found that autologous IPB patching significantly decreases the incidence of pneumothorax, chest tube placement, and hospital admission. There are several documented alternatives to blood patching that are utilized in effort to avoid pneumothorax complications including hydrogel plugs, fibrin glue, collagen foam plugs, absorbable haemostat gelatin powder, injection of normal saline tract sealant and even techniques like patient breath-hold after deep exhalation before needle extraction; however widespread use of a particular method has not yet been adopted.
A study comparing 318 patients who received hydrogel to 1,956 patients who received no intervention found that the treated group had a significantly lower incidence of pneumothorax (20.8 vs. 32.8%, P=0.001) and chest tube placement (8.2 vs. 20.8%, P<0.0001) (9). Fibrin glue has been studied as well, showing a decrease in the incidence of pneumothorax (40.6% to 19.2%) and chest tube placement from (18.8% to 3.8%, P<0.0025) (10). A prospective study of 50 patients utilizing collagen foam found a reduction in the incidence of pneumothorax with 8% (2 of 25) in the treated group compared to 28% (7 of 25) in the control group (11). A retrospective study investigating the efficacy of absorbable hemostat gelatin powder has demonstrated a reduction in the incidence of pneumothorax (8.8% vs. 21%; P=0.007) and chest tube placement (4% vs. 8.1%; P=0.195) in the treated group compared to the control (12). A prospective, randomized, controlled trial enrolling 322 patients studying normal saline intraparenchymal patching found a decrease in the incidence of pneumothorax (6.2% vs. 26.1%; P<0.001) and chest tube placement (0.6% vs. 5.6%: P=0.010) (13). A prospective, randomized controlled trial consisting of 407 patients undergoing lung biopsy also demonstrated noninferiority of the use of IBP compared to hydrogel with 2 hours post-biopsy pneumothorax rates being 21% and 29% and chest-tube placement rates as 9% and 13% for the blood patching and hydrogel groups, respectively (14).
Though alternatives have shown to decrease the rate of pneumothorax, blood patching has unique advantages with very little cost. As the blood is autologous, there is no risk of introduction of exogenous material into the patient’s body. Autologous blood is readily available if the patient is consenting and venous access is possible. Moreover, our study shows a decrease in not only the incidence (37% to 14.6%) of pneumothorax, but also in the severity (16% to 9%), need for chest tube placement (17.2% to 3.9%), and the hospitalizations rate (16.4% to 2.9%) suggesting that blood patching is a safe and effective technique that can be considered when performing percutaneous CT-guided lung biopsies.
A few limitations of this retrospective analysis should be noted. Firstly, some factors with a possible impact on the pneumothorax rate were not compared between the two groups, including lesion size, lesion location, and patient positioning. Secondly, all CT-guided lung biopsies using blood patching were performed by the same interventionist, whose level of expertise may differ from other interventionalists’ in the study. Thirdly, evaluation of subpleural emphysema or honeycomb formation in patients without COPD was beyond the scope of this study and may influence the likelihood of developing pneumothorax after CT guided needle biopsy (CTNB). Another point to consider is that this is a retrospective study and any differences found could be due to the reason the patient received the blood patch and not necessarily to the blood patch itself. Caution should be exercised when drawing conclusions from retrospective, observational, and non-controlled studies. Lastly, additional multivariate analyses maybe needed to perform to adjust for any potential imbalances which were beyond the scope of this study.
Conclusions
This study demonstrates that tract injection of autologous blood following images guided lung biopsy is a safe and effective method of reducing the incidence and severity of pneumothorax, and incidence of chest tube placement and hospitalization.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-9/rc
Data Sharing Statement: Available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-9/dss
Peer Review File: Available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-9/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-9/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board of Ascension St. John Hospital (IRB No. 1589530) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Heerink WJ, de Bock GH, de Jonge GJ, et al. Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur Radiol 2017;27:138-48. [Crossref] [PubMed]
- Bourgouin PM, Shepard JA, McLoud TC, et al. Transthoracic needle aspiration biopsy: evaluation of the blood patch technique. Radiology 1988;166:93-5. [Crossref] [PubMed]
- Herman SJ, Weisbrod GL. Usefulness of the blood patch technique after transthoracic needle aspiration biopsy. Radiology 1990;176:395-7. [Crossref] [PubMed]
- Malone LJ, Stanfill RM, Wang H, et al. Effect of intraparenchymal blood patch on rates of pneumothorax and pneumothorax requiring chest tube placement after percutaneous lung biopsy. AJR Am J Roentgenol 2013;200:1238-43. [Crossref] [PubMed]
- Graffy P, Loomis SB, Pickhardt PJ, et al. Pulmonary Intraparenchymal Blood Patching Decreases the Rate of Pneumothorax-Related Complications following Percutaneous CT-Guided Needle Biopsy. J Vasc Interv Radiol 2017;28:608-613.e1. [Crossref] [PubMed]
- Manhire A, Charig M, Clelland C, et al. Guidelines for radiologically guided lung biopsy. Thorax 2003;58:920-36. [Crossref] [PubMed]
- Collins CD, Lopez A, Mathie A, et al. Quantification of pneumothorax size on chest radiographs using interpleural distances: regression analysis based on volume measurements from helical CT. AJR Am J Roentgenol 1995;165:1127-30. [Crossref] [PubMed]
- Theilig D, Petschelt D, Mayerhofer A, et al. Impact of quantitative pulmonary emphysema score on the rate of pneumothorax and chest tube insertion in CT-guided lung biopsies. Sci Rep 2020;10:10978. [Crossref] [PubMed]
- Ahrar JU, Gupta S, Ensor JE, et al. Efficacy of a Self-expanding Tract Sealant Device in the Reduction of Pneumothorax and Chest Tube Placement Rates After Percutaneous Lung Biopsy: A Matched Controlled Study Using Propensity Score Analysis. Cardiovasc Intervent Radiol 2017;40:270-6. [Crossref] [PubMed]
- Petsas T, Siamblis D, Giannakenas C, et al. Fibrin glue for sealing the needle track in fine-needle percutaneous lung biopsy using a coaxial system: Part II--Clinical study. Cardiovasc Intervent Radiol 1995;18:378-82. [Crossref] [PubMed]
- Engeler CE, Hunter DW, Castaneda-Zuniga W, et al. Pneumothorax after lung biopsy: prevention with transpleural placement of compressed collagen foam plugs. Radiology 1992;184:787-9. [Crossref] [PubMed]
- Baadh AS, Hoffmann JC, Fadl A, et al. Utilization of the track embolization technique to improve the safety of percutaneous lung biopsy and/or fiducial marker placement. Clin Imaging 2016;40:1023-8. [Crossref] [PubMed]
- Li Y, Du Y, Luo TY, et al. Usefulness of normal saline for sealing the needle track after CT-guided lung biopsy. Clin Radiol 2015;70:1192-7. [Crossref] [PubMed]
- Maybody M, Muallem N, Brown KT, et al. Autologous Blood Patch Injection versus Hydrogel Plug in CT-guided Lung Biopsy: A Prospective Randomized Trial. Radiology 2019;290:547-54. [Crossref] [PubMed]
Cite this article as: Jain N, Zurla Z, Crowley S, Gupta N, Bajaj S, Khanna K. Tract injection of autologous blood (intraparenchymal blood patching) in percutaneous transthoracic CT-guided lung biopsy and the incidence of pneumothorax: a retrospective analysis. Precis Cancer Med 2022;5:34.

