
Moving through rare lung cancer histologies: a narrative review on diagnosis and treatment of selected infrequent entities
Introduction
Lung cancer is the second most common malignancy and the first cause of cancer death in both sexes worldwide (1). According to the 2021 World Health Organization (WHO) Classification of thoracic tumors, more than 85% of advanced non-small cell lung cancers (NSCLC) belong to four main histologies: squamous cell carcinoma (SCC), adenocarcinoma (ADC), large cell carcinoma (LCC) and small cell lung carcinoma (SCLC) (2), while the remaining 5% corresponds to NSCLCs with a rare histology. These recent trends reflect the efforts over the past decade in the accurate determination of histological subtypes, as well as the introduction of immunohistochemistry (IHC) especially for those poorly differentiated NSCLCs not otherwise specified (NOS) in bioptic or resected samples (3,4). The precise determination of the histological subtype is of great importance because it has treatment and outcome implications (5,6). Moreover, in recent years, the broad spread of powerful diagnostic technologies, such as Next Generation Sequencing (NGS) techniques, has brought to a deeper understanding of molecular mechanisms underlying lung neoplastic transformation, paving the way for a personalized medicine even for rare lung neoplasms (7).
Due to the low incidence of these cancers, there is a lack of randomized prospective trials and reliable data to guide the best treatment choice. Indeed, most of the evidence derives from retrospective studies, subgroup analysis of clinical trials involving different histologies, or is borrowed from the treatment guidelines of their more common counterparts.
In this review, we aim at summarizing diagnostic tools, clinical characteristics, and main treatment principles of most rare lung epithelial neoplastic entities, excluding large cell carcinoma (even if it is an entity becoming very rare in its “null” phenotype, it has therapeutic options similar to the other more frequent histotypes) and those of neuroendocrine origin, in order to help physicians in everyday clinical practice. Thoracic sarcomas are not an aim of this review because of their different clinical management and treatment. We present the following article in accordance with the Narrative Review reporting checklist (available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-4/rc).
Methods
We compiled a narrative review on clinical, pathological and therapeutic aspects of the main rare lung cancer histologies according to the 2021 WHO Classification (Table 1). The scientific articles (published from 2010 onwards, full-text and in English language) were selected by searching through two of main online scientific databases, PubMed and EmBase. The main keywords searched were: “lung cancer AND rare histologies, “pulmonary sarcomatoid carcinoma”, “adenosquamous lung carcinoma”, “primary salivary gland-type lung tumor”, “NUT carcinoma”, “SMARCA4-deficient thoracic tumors”. For additional details, see supplementary appendix (Table S1).
Table 1
| Type | Subtype | Frequency | Histology | Immunohistochemistry | Genetic alterations | Treatment options |
|---|---|---|---|---|---|---|
| Pulmonary sarcomatoid carcinoma | Pleomorphic carcinoma | 2–3% | NSCLC with at least 10% of spindle and/or giant cells, or carcinoma consisting entirely of spindle and/or giant cells | Variably reactive or negative for panCK (spindle/giant cell component) | TP53 and KRAS mutations; less frequently alterations in genes such as EGFR, ALK, BRAF, STK11, NOTCH1, NRAS, PI3KCA, CDKN2B, CKDN2A, MET, RET, HER2 and NF1 | Surgery; platinum-based chemotherapy; targeted therapies, i.e., MET inhibitors for MET exon 14 skipping mutations, KRAS G12C inhibitors for p.G12C KRAS mutations; ICIs |
| Carcinosarcoma | <0.2% | NSCLC elements with sarcomatous heterologous component | Sarcomas’ markers, i.e., desmin, myogenin (sarcomatous component) | |||
| Pulmonary blastoma | <0.1% | Fetal-type adenocarcinoma and primitive mesenchymal stroma | Vimentin+ (mesenchymal component) | |||
| Adenosquamous lung cancer | 2–3% | Tumor having ≥10% components of both ADC and SCC | TTF1 and napsin A for ADC, p40 for SCC | EGFR, KRAS, BRAF mutations | Surgery; platinum-based chemotherapy; targeted therapies, i.e., EGFR inhibitors; ICIs | |
| Primary salivary gland-type lung tumor | Adenoid cystic carcinoma | 0.1–0.2% | Biphasic tumor composed of epithelial and myoepithelial cells growing with tubular, cribriform or solid pattern | CK, vimentin, actin, S100, CD117 | Surgery; platinum-based chemotherapy | |
| Mucoepidermoid carcinoma | Mixture of squamous cells, mucin-secreting cells, and intermediate-type cells | CK+; S100, SMA, TTF1 and napsin A−; p40, p63, CK5/6+ in the squamoid cells | ||||
| Epithelial-myoepithelial carcinoma | Inner layer of cuboidal epithelial cells forming duct-like structures, peripherally bounded by an outer layer of myoepithelial cells | CK+ (epithelial cells); S100, p40, p63, actin (myoepithelial cells) | ||||
| NUT carcinoma | Currently unknown | Mixture of cells composed of more differentiated pattern with focal keratinization and sheets/nests of smaller undifferentiated cells with monomorphic appearance | CK5/6, p63+, p40+/−, CD34+/−, staining with clone C52B1 (highly specific rabbit anti-human monoclonal NUT antibody); occasionally chromogranin, synaptophysin, TTF1+ | NUTM1 gene rearrangement (genes partners: 70% BRD4, 30% BRD3, NSD3 and others) | Radiotherapy (surgery); chemotherapy (no standard regimens, mainly combinations of platinum, taxane, anthracyclin); BET-inhibitors | |
| SMARCA4-deficient thoracic tumor | Diffuse sheets of variably discohesive, large round to epithelioid cells with vesicular chromatin and prominent nucleoli. Generally monomorphism appearance. Rhabdoid cells may be present | CK low or absent; CD34, SOX2, SALL4 +; SMARCA4 − (or low +); SMARCA2 –; SMARCB1 + | BRG/BRM loss | Surgery; anthracycline-based chemotherapy; ICIs; BET and EZH2 inhibitors |
ADC, adenocarcinoma; SCC, squamous cell carcinoma; NSCLC, non-small cell lung cancer; CK, cytokeratin, panCK, pancytokeratin; ICIs, immunocheckpoint inhibitors; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; BRAF, B-Raf proto-oncogene; STK11, serine/threonine kinase 11; NOTCH1, Notch homolog 1, translocation-associated; NRAS, neuroblastoma ras viral oncogene homolog; KRAS, Kirsten rat sarcoma virus; PI3KCA, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha, CDKN2B, cyclin dependent kinase inhibitor 2B, CKDN2A, cyclin dependent kinase inhibitor 2A, MET, MET proto-oncogene, receptor tyrosine kinase; RET, rearranged during transfection; HER2, human epidermal growth factor receptor 2; NF1, neurofibromatosis 1; TP53, tumor protein p53; BET, bromodomain and extraterminal protein; EZH2, enhancer of zeste homolog 2; NUT, nuclear protein in testis; NUTM1, NUT midline carcinoma family member 1; BRD4, bromodomain containing 4; BRD3, bromodomain containing 4; NSD3, nuclear receptor SET domain-containing 3; BRM, Brahma gene; BRG, Brahma-related gene.
Pulmonary sarcomatoid carcinoma (PSC)
PSC are a rare and poorly differentiated subtype of NSCLC, showing features suggestive of both epithelial and mesenchymal differentiation.
The 2021 WHO classification of lung tumors distinguishes 3 PSC categories: pleomorphic carcinoma, accounting for 2–3% of all NSCLC cases in surgical series, carcinosarcoma, representing <0.2% of lung cancers and pulmonary blastoma constituting <0.1% of all NSCLCs (2).
Pleomorphic carcinoma is defined as a poorly differentiated NSCLC (namely, ADC, SCC, and/or LCC) composed of at least 10% of spindle and/or giant cells, or a carcinoma consisting entirely of spindle and/or neoplastic giant cells. In some cases, the epithelial and the spindle/giant cell components are found to be intimately admixed, while other times they appear clearly delimited from one another. Necrosis, haemorrhage, and vascular invasion are common findings, and a collagenous or myxoid stroma is usually present in variable amounts. Spindle cell carcinoma and giant cell carcinoma are classified as two subtypes of pleomorphic carcinoma, the former being solely composed of neoplastic spindle cells, and the latter consisting entirely of markedly pleomorphic giant cells (Figure 1) (2).
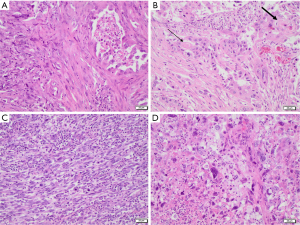
Carcinosarcoma is a tumor showing a combination of NSCLC elements and at least one sarcomatous heterologous component (osteosarcoma, rhabdomyosarcoma, chondrosarcoma, liposarcoma, angiosarcoma). A minimum percentage of the minority component is not required for diagnosis (2).
Pulmonary blastoma is a biphasic tumor composed of fetal-type adenocarcinoma and primitive mesenchymal stroma. The epithelial component usually resembles endometrioid carcinoma, consisting in glandular structures composed of columnar cells with small uniform nuclei. In a minority of cases the glands have more atypical features, being similar to conventional ADC. The mesenchymal component consists of oval or spindle-shaped small blastema-like cells and more mature fibroblast-like cells (2).
Since, by definition, pleomorphic carcinoma needs to have ≥10% of spindle and/or giant cell components, it cannot be diagnosed on small biopsies, but it requires a surgically resected specimen. On the other hand, the diagnosis of pulmonary blastoma or carcinosarcoma can be made also on small biopsies, as long as both the glandular and sarcomatous components are present and well recognizable in the specimen.
Most PSCs can be recognized from their morphological features only, while IHC stains may help to identify the different cell components in tumor tissues. Usually, the carcinomatous component stains with pancytokeratin and with different epithelial markers, depending on its direction of differentiation (TTF1 and napsin A for ADC, p40 for SCC). The spindle and giant cell components of pleomorphic carcinoma are variably reactive or negative for pancytokeratin. The mesenchymal component of pulmonary blastoma expresses vimentin, while the sarcomatous components of carcinosarcoma can be highlighted using markers such as desmin and myogenin for rhabdomyosarcoma, or S100 for chondrosarcoma.
Compared to other histologic subtypes, PSCs have an aggressive behaviour associated with poor prognosis, even at early stages (8). Even if surgery is considered the most effective therapy, radically resected patients have a shorter median overall survival (OS) as compared to common NSCLC ones (24 vs. 42 months) (9). Indeed, PSCs often relapse after radical surgery and they are generally refractory to conventional chemotherapy and radiotherapy. A significant proportion of patient has advanced disease at diagnosis (9). Among these, subjects receiving first-line platinum-based chemotherapy generally experience a median progression free survival (PFS) and OS of about 2 and 6 months, respectively (9,10).
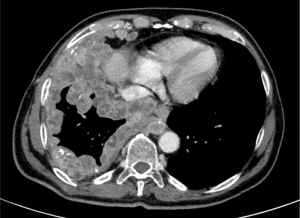
Most patients with PSC are male, former or current smokers, with a median age at diagnosis of 65 years. An exception is pulmonary blastoma, which usually affects younger patients, indifferently of gender (11,12). Clinical symptoms do not differ from those of other lung cancers, being related to tumor size and location and including cough, haemoptysis and chest pain (11). Giant cell carcinoma may produce G-CSF or beta-hCG, leading to fever, neutrophilia and gynecomastia (13,14). Radiologically, PSC may present as a large peripheral mass with rounded and well-defined margins, or as endobronchial polypoid lesions (Figure 2). PSCs tend to metastasize not only to usual sites (i.e., liver, brain, bone, adrenal glands) but also to less common ones (i.e., kidney, retroperitoneum, small bowel and pancreas).
Since common mutations have been found both in the carcinomatous and in the sarcomatous components, the origin of PSC is probably clonal, linked to alterations in genes involved in epithelial-mesenchymal transition (EMT) (15). Interestingly, EMT, which is characterized by increased vimentin expression and decreased E-cadherin expression along with histologic transformation, is one of the resistance mechanisms of common NSCLC during treatment with chemotherapy or tyrosine kinase inhibitors (TKIs) (16).
In the last years, molecular characterization of PSC has been object of research. According to recent studies, the most frequent mutations occur in TP53 and KRAS genes. Less frequently, PSC could harbour various types of alterations in genes such as EGFR, ALK, BRAF, STK11, NOTCH1, NRAS, PI3KCA, CDKN2B, CKDN2A, MET, RET, HER2 and NF1 (17-19). Few studies reported EGFR mutation positive PSC patients treated with EGFR inhibitors with disappointing results (20,21), suggesting that EGFR mutations in these tumors may be more likely a secondary event than an essential pathogenetic driver. Interestingly, a higher frequency of MET exon 14 skipping mutations (ranging from 9.5% to 22%) has been described in PSC and seems to be mutually exclusive with other targetable genetic alterations such as EGFR and BRAF mutations as well as ALK and ROS1 rearrangements (22,23). Many pre-clinical and clinical trials showed interesting response rates to small molecules targeting MET (i.e., crizotinib, savolitinib, cabozantinib), suggesting that MET could be an effective target in PSC (24,25). The GEOMETRY trial (NCT02414139) evaluated the efficacy of capmatinib, a small molecule kinase inhibitor targeting MET, at the dose of 400 mg bid in 97 patients with metastatic NSCLC and confirmed MET exon 14 skipping mutation. The overall response rate (ORR) was 68% (4% complete response) among the 28 treatment-naïve patients and 41% (all partial response) among the 69 previously treated patients. In this study, only one patient with a histology other than ADC, SCC or LCC has been enrolled. The VISION trial evaluated the efficacy and safety of tepotinib, a highly selective MET inhibitor, at a dose of 500 mg once daily in 152 patients with advanced or metastatic NSCLC (only 3 patients had sarcomatoid features) with a confirmed MET exon 14 skipping mutation. Among the 99 treated patients with at least 9 months of follow-up, the ORR was 46% (all partial response), according to independent review (26). Finally, savolitinib, another highly selective oral MET TKI, was tested in 70 patients with unresectable or metastatic MET exon 14 skipping mutated PSC (25/70) and other NSCLC, with an ORR of 50% (all partial response) in the PSC subgroup (27).
Last but not least, the preclinical and clinical success of specific KRAS inhibitors may lead to interesting results even in this subgroup (28,29). In the phase II CodeBreak 100 trial, sotorasib, a first-in-class small molecule that specifically and irreversibly inhibits p.G12C mutated KRAS, showed responses in more than a third of the 126 patients with locally advanced or metastatic NSCLC harbouring KRAS G12C mutations progressed on no more than three prior standard therapies. Median PFS and median DoR were 6.8 months and 10 months, respectively (30). The CodeBreak 200 is an ongoing, randomized, phase 3 study evaluating sotorasib versus docetaxel for the treatment of previously treated locally advanced and unresectable or metastatic NSCLC patients with p.G12C mutated KRAS (NCT04303780).
Of note, PSCs are characterized by high tumor mutational burden (TMB), especially those with TP53 and KRAS mutations (17,18). Moreover, studies have demonstrated that PD-L1 overexpression is common in PSC, ranging from 53% to 90% of all cases (10,31-33). Although neither TMB nor PD-L1 is a perfect predictive biomarker for immunotherapy in lung cancer, several reports suggest the possible activity of immune checkpoint inhibitors directed against programmed death 1 (PD-1) or its ligand-1 (PD-L1). However, conclusive data are lacking as these patients were not included in prospective studies (34-40). Results from ongoing trials prospectively exploring these agents in patients with rare lung tumors, such as the CHANCE trial (NCT03976518), are awaited.
Adenosquamous lung cancer (ASC)
ASC is a relatively rare histotype of NSCLC accounting for approximately 2–3% of all lung cancers (2). Prior to 1999 this entity was not well defined and ASC was simply described as a mixture of squamous cell carcinoma (SCC) and adenocarcinoma (ADC). Nowadays, according to the WHO 2021 classification, ASC is defined as a tumor having 10% components of both ADC and SCC (2,41).
Histological diagnosis of ASC may be straightforward if the ADC and SCC components are well differentiated and represented, with the former having a glandular morphology and the latter being keratinizing. Otherwise, if the tumor has a non-keratinizing SCC component and/or a solid adenocarcinoma component, diagnosis may be more difficult. In this case, immunohistochemistry is of great help, as each component maintains the immunoprofile of its conventional counterpart (i.e., TTF1 and napsin A for ADC, p40 for SCC). Co-expression of both ADC and SCC markers in the same tumor cells is a rare phenomenon and it does not support the diagnosis of ASC (2).
Due to the heterogeneity of ADC and SCC components within the tumor mass, it is not possible to diagnose ASC on small biopsies or cytology specimens, but it is necessary to have a resected specimen showing ≥10% of each component. The identification of both components depends on the extent of histological sampling of the tumor. However, if a small biopsy shows both ADC and SCC components, the possibility of ASC should be considered and commented in the pathology report (2) (Figure 3).
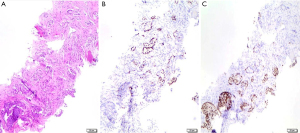
ASC is not simply a mixture of ADC and SCC, but it represents a distinct histological entity with worse prognosis than ADC and SCC (42-44). Some studies have observed that ASC is prevalent in men (42,44-46) and smokers (44,47-49). Conflicting opinions are reported in literature regarding the mean age of onset, as some report an average age of onset of 60.8 years (±9.9; P=0.007) while others suggest that ASC patients are younger than ADC and SCC patients (44).
Unlike ADC and SCC, the origin and pathogenesis of ASC is still subject of debate. Three theories have been hypothesized. The collision theory assumes that ASC is the product of collision between two separate and independent foci of ADC and SCC which, as they grow, would develop a single entity containing the two components. The second theory suggests the histological transition between ADC and SCC. Indeed, Tsuhako et al. found Human Papilloma Virus (HPV) DNA in cells of 23 surgically resected ASC patients. On microscopic analysis, ADC cells adjacent to SCC cells were larger in size and stained positive for high molecular weight cytokeratins, demonstrating the possibility of transition between different histotypes (50). A study by Kanazawa et al. found high expression of squamous cell antigens and low expression of MUC1 (typical of glandular differentiation) in ADC cells of ASC (51). The third theory states that ASC derives from an undifferentiated bipotential common precursor that differentiates both in ADC and SCC during the course of the disease. Shi et al. investigated the frequency of major driver oncogenes in 56 ASC patients. Microdissection or macrodissection was performed in 37 cases to split ADC and SCC components and sequence them separately with NGS, polymerase chain reaction (PCR), and Sanger sequencing. The authors found that the major driver oncogenes (EGFR, KRAS and BRAF) were identical in both components, supporting the clonal origin (52). Lin et al. studied the genomic origin of ASC collecting 227 resected tumor samples, showing that 42.7% genomic alterations were shared, with no differences on mutated genes comparing ADC and SCC components (53).
ASC is characterized by a more aggressive behavior and worse prognosis compared to pure ADC and SCC. This could be, at least in part, due to the higher stage of ASC at presentation as compared to ADC and SCC (Figure 4). Indeed, different studies showed a 5-years OS rate between 23.3% and 42% (stage IA) in radically resected patients (44,45,48,54). However, registry data show that ASC patients are more likely to receive surgery as compared to ADC and SCC (50.4% vs. 32.5% and 29.1%, respectively; P<0.001). On the other side, they are less frequently treated with radiation therapy or chemotherapy (55).
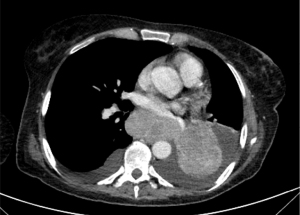
Focusing on molecular targeted treatment, retrospective series of Asian patients showed that approximately 30% to 50% of ASCs harbor EGFR activating mutations. The median PFS on first-generation TKIs range from 9.3 to 10.1 months (53,56,57). In a small study evaluating 16 Caucasian patients, neither MET mutations nor ALK-ROS1 rearrangements were detected at NGS and FISH analysis. Interestingly, EGFR mutations were more prevalent than expected for a Caucasian population (5 out of 16 tumors) (58).
Liu et al. investigated heterogeneity of PD-L1 expression in 72 resected ASC patients. Thirty-five of 72 patients were found positive for PD-L1 expression with a 5% cut-off; 20.8% of patients were positive for PD-L1 expression in ADC components and 34.7% of patients were positive for PD-L1 expression in SCC components. The two components showed a discordance rate in PD-L1 expression in 51.9% demonstrating a high tumor heterogeneity (59). Consistent results were reported by Shi et al. in another study of 51 patients where the positivity rate was 11.11% in ADC component and 38.89% in SCC component (cut-off 25%) (60). It is unclear whether the heterogeneity of PD-L1 expression in the two components of ASC may compromise the effectiveness of immunotherapy treatments. Notably, ASC were excluded from registration studies of ICIs in NSCLC.
Primary salivary gland-type lung tumor
Salivary gland-type tumors can originate from any exocrine glandular tissue, with the most common site being the head and neck region. According to the 2021 WHO classification, primary salivary gland-type lung tumors (p-SGTs) are a rare and distinct group of neoplasms accounting for approximately 0.1–0.2% of lung cancers (2). p-SGTs are histologically indistinguishable from their counterparts in the salivary gland and are thought to originate from the submucosal glands of the tracheobronchial tree (61). The most common histotypes are adenoid cystic carcinoma (ACC), mucoepidermoid carcinoma (MEC), and epithelial-myoepithelial carcinoma (EMC), while other histologies are less frequent (62,63).
ACC is a biphasic malignant tumor composed of epithelial and myoepithelial cells. Architecturally, it shows three main growth patterns: tubular, cribriform, and solid. The most frequent pattern is the cribriform, characterized by abnormal nests of epithelial cells with cylindromatous microcystic spaces filled with hyaline or basophilic myxoid material, surrounding and/or infiltrating glandular structures within the lung parenchyma (Figure 5). The presence of both epithelial and myoepithelial components can be demonstrated using immunohistochemistry stains such as cytokeratins, vimentin, actin, S100, and CD117.
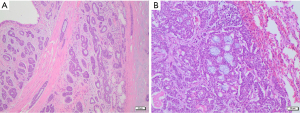
MEC consists of a mixture of squamous cells, mucin-secreting cells, and intermediate-type cells, with a variable presence of macrocysts and cellular pleomorphism depending on tumor grade (64,65). Most of the tumors are of low grade, showing cystic spaces lined by mucin-secreting cells and rare mitoses. High-grade tumors are less common, they mainly show solid areas composed of atypical squamous cells and intermediate-type cells, with frequent mitoses, necrosis, and a variable number of mucin-secreting cells. All cell types in MECs stain positive for epithelial markers, such as cytokeratins, and negative for mesenchymal markers, like S100 and SMA. Adenocarcinoma markers (TTF1, napsin A) are always negative, while SCC ones (p40, p63, CK5/6) are positive in the squamoid cells.
EMC is pathologically characterized by two cell populations: an inner layer of cuboidal epithelial cells forming duct-like structures, peripherally bounded by an outer layer of myoepithelial cells (66). Three main histological patterns have been reported. The most common one shows duct-like structures formed by epithelial cells with small nuclei and eosinophilic cytoplasms and outer myoepithelial cells, which often display a clear cytoplasm. The second most common pattern is characterized by solid areas formed by eosinophilic and spindled myoepithelial cells. The last pattern, in terms of frequency, shows a predominance of atypical myoepithelial cells and is associated with a worse prognosis. The two different cellular components can be highlighted using immunohistochemistry for epithelial (cytokeratins) and myoepithelial cells (S100, p40, p63, actin).
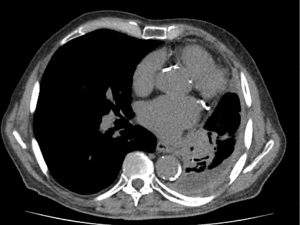
In terms of risk factors, smoking does not appear to be associated with the onset of EMC, while a higher percentage of non-smokers have been reported in MEC patients than those diagnosed with ACC (67,68). No gender differences have been reported (61,69,70). p-SGTs usually present as central lesions and are associated with radiological characteristics similar to those of post obstructive pneumonia and/or atelectasis (69) (Figure 6). Although p-SGTs are usually considered as slow growing indolent tumors, some aggressive cases have been reported. Compared to ACC, patients with MEC tend to be younger and with earlier disease stage (61,69).
Most literature data derive from retrospective series (65,69,71). Surgery represents the only treatment that significantly improves p-SGTs patients’ survival, with 5-years survival rates between 63.4% and 97.6% in different series including patients with different histologies and disease stages. Pathological grade may be the only independent prognostic factor in resected MEC, while nodal involvement is not (65,71). Moreover, lymphadenectomy does not seem to improve survival as well as adjuvant radiotherapy (65,69).
Surgery may also be the best treatment choice for disease recurrences, given the limited activity of radiotherapy (69,71).
Chemotherapy has very low activity in p-SGTs, however it can be considered in patients with inoperable disease. The most common regimens include cisplatin/carboplatin plus paclitaxel, cisplatin plus vinorelbine, and cisplatin plus gemcitabine, although few partial responses have been reported (72,73). No predictive molecular pathology was described for these entities.
NUT carcinoma (NC)
NC is a rare, poorly differentiated carcinoma, genetically defined by the presence of nuclear protein in testis (NUTM1) gene rearrangement. Although it can arise in any part of the body, the most common site is lung (35.3%), followed by head and neck region (35%) and mediastinum (26%).
NC can be composed of a mixture of two distinct cell populations: the first shows a more differentiated pattern with focal keratinization, while the second consists of sheets and nests of smaller undifferentiated cells with monomorphic appearance. Mitoses and necrosis are often present, as well as a prominent neutrophilic infiltrate admixed with tumor cells. Focal keratinization may be present in 33% of cases. Overall, the histologic appearance of NUT, being that of an undifferentiated carcinoma, is not specific, and demonstration of NUTM1 rearrangement is required for diagnosis. This can be achieved through IHC, using a highly specific rabbit anti-human monoclonal NUT antibody (clone C52B1), due to the overexpression of the NUT protein in neoplastic cells. The staining is typically punctuated and a >50% nuclear positivity is diagnostic. Generally, NUT expression is higher in the population of undifferentiated cells, as opposed to the keratinized population that shows low or absent protein expression. Both components usually show CK 5/6 and p63/p40 expression, though a loss of p40 expression in the keratinized tumor cell population has been reported in some cases. NCs often stain for CD34 and occasionally they show positivity also for chromogranin, synaptophysin, and even TTF1 (74-78) (Figure 7).
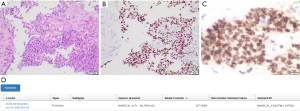
Due to its characteristics, NC may be difficult to diagnose on tissue biopsies, as sometimes small specimens include only one of the two cell populations, resulting in misdiagnosis.
NUT rearrangement could be detected by other techniques such as FISH, PCR, and NGS (79). Indeed, NC is characterized by an acquired chromosomal rearrangement involving NUT midline carcinoma family member 1 (NUTM1) gene on chromosome 15q14. Unlike other carcinomas that show complex karyotypes, NC often shows a simple karyotype with only one aberration. About 70% of NCs present a reciprocal translocation between NUTM1 and BRD4 (BET protein family encoding gene) on chromosome 19p13.12 (80-82). In the remaining 30% of cases NUTM1 shows BRD3, NSD3 and other unspecified genes as partners (83,84).
NC usually involves subjects in their second or third decade of life, with high incidence in pediatric age (85). Males and females are equally affected (81,85). NC is a highly aggressive tumor that often presents with both early local invasion and distant metastases (51%) (81) (Figure 8). NC has not been associated with any known risk factor (86).
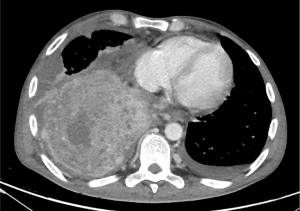
To date, there are no highly effective treatments for NC. A systematic review including 64 publications and 119 patients showed that surgery did not significantly improve patient outcomes [1 year OS surgery group 33.8% (95% CI: 14.2–54.88%) vs. 19.7% (95% CI: 10.98–30.38%); 5-year OS <5% in both groups]. The use of chemotherapy improved OS only in mediastinal NC (P=0.0288). Radiotherapy, with a dose higher than 50 Gray, improved OS [1- and 2-year OS of 24.99% (95% CI: 16.31–34.64%) and 9.45% (95% CI: 4.01–17.72%), respectively]. Median OS was 5 months. The different NUT rearrangement partners were not associated with outcomes (85).
BET inhibitors are a class of drugs that bind and inhibit the BRD3/4 portion of BRD3/4-NUT preventing the interaction with acetylated lysines of histones and transcription factors. These drugs demonstrated activity in preclinical xenograft models of NC leading to rapid squamous differentiation and growth disruption on tumor cells (87). The efficacy of these drugs in NC patients has been investigated in several phase I trials showing low response rates (88,89). Similar results were obtained from the use of histone deacetylase inhibitors (90). Recently, preclinical models suggested that the combination of a BET inhibitor with a selective p300/CBP histone acetyltransferase may provide greater growth inhibition as compared to single-agent BET inhibitor or chemotherapy (91).
Based on the available evidence, it appears that early radiation therapy may be considered the primary treatment of NC. Surgical resection could be an option in selected patients where the intent is radical, while chemotherapy should be considered especially as a part of integrated treatments, although there is not a standard chemotherapy regimen. Case reports of patients treated with different schemes for advanced NC of different origin have been reported, including dose-dense paclitaxel plus ifosfamide plus cisplatin, vincristine plus cyclophosphamide plus doxorubicin sometimes alternated with ifosfamide plus etoposide, carboplatin plus nab-paclitaxel (92-96).
Thoracic SMARCA4 deficient undifferentiated tumors (DUTs)
This is a newly described category of undifferentiated tumor characterized by high-grade malignancy and deficiency of SMARCA4, a key member of the BAF chromatin-remodelling complex (2).
SMARCA4-DUTs consist of diffuse sheets of variably discohesive, large round to epithelioid cells with vesicular chromatin and prominent nucleoli. The appearance is characterized by a relatively monomorphism with occasional cells displaying mild to moderate pleomorphism. Rhabdoid cells may be present. Mitoses and necrosis are common. Unequivocal evidence of epithelial differentiation is absent and cytocheratin are often expressed weakly or may be negative. Many cases express CD34, SOX2 and or SALL4 as staminal markers. Complete loss of SMARCA4 (BRG) expression is typical, but about 25% of cases could show reduction of SMARCA4 staining. SMARCA2 (BRM) staining is lost in most cases while SMARCB1 (INI1) expression is retained.
SMARCA4-DUTs are neoplasms with very aggressive clinical behavior with a median OS between 4 and 7 months and a 2-year survival rate of 12.5% (97-102). They generally affect young patients between 30 and 59 years. There is a strong male predominance with a male to female ratio of 9:1 and most patients are heavy smokers (97-99,101,103).
At diagnosis, patients usually present with large compressive and infiltrative chest masses causing chest pain, dyspnoea and superior vena cava syndrome (98).
Commonly, the primary tumor has a thoracic location with an average size of 10 cm (100) (Figure 9). Radiological studies have identified four common patterns of presentation of SMARCA4-DUTs, of which the most frequent is the mediastinal pattern, followed by the pleural, cervical and retroperitoneal patterns with rates of 61%, 29%, 5% and 5%, respectively (98,103).
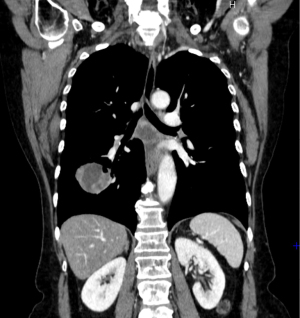
Similar to NSCLC, metastatic involvement at diagnosis is very common, involving mainly lymph nodes, adrenal glands, lung, bone and brain (98,103).
To date, there is no approved treatment strategy for SMARCA4-DUTs. Similarly to soft tissue sarcomas, first line therapy can be anthracycline-based chemotherapy, although reported efficacy rates are extremely low and no standard schemes are approved. Even when tumors are radically resected, the recurrence rates are extremely high (97,98).
In light of the promising results of immunotherapy use, especially PD-1 inhibitors, in certain subgroups of patients with soft tissue sarcoma (104-107), immunocheckpoint inhibitors were tested in SMARCA4-DUTs patients as well. Gantzer et al. (108) analyzed tumor microenvironment of 9 patients diagnosed with SMARCA4-DUTs. Eight of these 9 patients had a tumor microenvironment devoid of efficient immune cells, with low levels of CD8-positive and CD3-positive lymphocytes. The most represented immune population was constituted by CD68-positive macrophages. Of these 9 patients, 4 received immunotherapy as part of their treatment but only one achieved a prolonged partial response with nivolumab/ipilimumab combination. Analysis of this patient’s tumor microenvironment showed the presence of a rich inflammatory infiltrate with high levels of CD8-positive and CD3-positive lymphocytes and the presence of TLS (tertiary lymphoid structures), ectopic lymphoid structures located close to the tumor, capable of generating an adaptive immune response (109-111). Although further studies on the role of TLS in the context of immunotherapy are needed, based on these early results and considering the rarity of SMARCA4-DUTs and their resistance to chemotherapy, presence of TLS may represent a guide to direct therapeutic choices in these patients in the next future.
However, although some successful experiences on immunotherapy, alone or in combination with chemotherapy, in SMARCA4-DUTs patients have been published, to date no solid therapeutic recommendation about the role of such agents can be made (107,112-115).
Preclinical and clinical studies with BET inhibitors and EZH2 inhibitors in patients with SMARCA4-DUTs are currently ongoing (NCT03213665; NCT02875548; NCT02601950), underlining the importance of early identification of these tumors in order to ensure a personalized treatment strategy, including enrollment in clinical trials (116,117).
Conclusions
Rare lung cancers diagnosis is often difficult, especially when dealing with small biopsy samples. This fact, along with their rarity, has limited the amount of evidence about the correct clinical management of these tumors. However, the evolution of diagnostic techniques, along with the increasing awareness about such rare entities, will probably lead to higher rate of diagnosis and, hopefully, prospectively collected clinical and experimental data. Genomic information may guide treatment decisions in some subsets of rare tumors too. For example, prospective clinical trials with targeted therapies against MET exon 14 skipping mutation positive sarcomatoid tumors are currently ongoing, with some promising early results. Moreover, as most of these entities are chemo-refractory, results from clinical studies exploring immune checkpoint inhibitors are eagerly awaited. Given the complexity and rarity of these tumors, a multidisciplinary discussion on both diagnostic and therapeutic strategies is of paramount importance.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-4/rc
Peer Review File: Available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-4/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-4/coif). SN reports personal fees (as speaker bureau or advisor) from Eli Lilly, MSD, Roche, BMS, Takeda, Pfizer, Astra Zeneca, Boehringer Ingelheim, Novartis, AMG and Sanofi, outside the submitted work. PB reports personal fees from Astra Zeneca, Bristol Myers Squibb, Roche, Takeda, Institutional research grant from Pfizer and Roche, travel expenses from Amgen and Daiichi Sankyo, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- WHO. WHO Classification of Tumours, 5th ed. 2021. Available online: https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/Thoracic-Tumours-2021
- Nicholson AG, Tsao MS, Beasley MB, et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J Thorac Oncol 2022;17:362-87. [Crossref] [PubMed]
- Yatabe Y, Dacic S, Borczuk AC, et al. Best Practices Recommendations for Diagnostic Immunohistochemistry in Lung Cancer. J Thorac Oncol 2019;14:377-407. [Crossref] [PubMed]
- Righi L, Vavalà T, Rapa I, et al. Impact of non-small-cell lung cancer-not otherwise specified immunophenotyping on treatment outcome. J Thorac Oncol 2014;9:1540-6. [Crossref] [PubMed]
- Ota T, Kirita K, Matsuzawa R, et al. Validity of using immunohistochemistry to predict treatment outcome in patients with non-small cell lung cancer not otherwise specified. J Cancer Res Clin Oncol 2019;145:2495-506. [Crossref] [PubMed]
- Ding Y, Shao Y, Na C, et al. Genetic characterisation of sarcomatoid carcinomas reveals multiple novel actionable mutations and identifies KRAS mutation as a biomarker of poor prognosis. J Med Genet 2022;59:10-7. [Crossref] [PubMed]
- Roesel C, Terjung S, Weinreich G, et al. Sarcomatoid carcinoma of the lung: a rare histological subtype of non-small cell lung cancer with a poor prognosis even at earlier tumour stages. Interact Cardiovasc Thorac Surg 2017;24:407-13. [PubMed]
- Rahouma M, Kamel M, Narula N, et al. Pulmonary sarcomatoid carcinoma: an analysis of a rare cancer from the Surveillance, Epidemiology, and End Results database. Eur J Cardiothorac Surg 2018;53:828-34. [Crossref] [PubMed]
- Vieira T, Girard N, Ung M, et al. Efficacy of first-line chemotherapy in patients with advanced lung sarcomatoid carcinoma. J Thorac Oncol 2013;8:1574-7. [Crossref] [PubMed]
- Pelosi G, Sonzogni A, De Pas T, et al. Review article: pulmonary sarcomatoid carcinomas: a practical overview. Int J Surg Pathol 2010;18:103-20. [Crossref] [PubMed]
- Sun L, Dai J, Chen Y, et al. Pulmonary Sarcomatoid Carcinoma: Experience From SEER Database and Shanghai Pulmonary Hospital. Ann Thorac Surg 2020;110:406-13. [Crossref] [PubMed]
- Yaturu S, Harrara E, Nopajaroonsri C, et al. Gynecomastia attributable to human chorionic gonadotropin-secreting giant cell carcinoma of lung. Endocr Pract 2003;9:233-5. [Crossref] [PubMed]
- Nonami Y, Yamamoto M, Sasaguri S. G-CSF producing giant tumor in the lung. J Cardiovasc Surg (Torino) 2005;46:313-4. [PubMed]
- Baldovini C, Rossi G, Ciarrocchi A. Approaches to Tumor Classification in Pulmonary Sarcomatoid Carcinoma. Lung Cancer (Auckl) 2019;10:131-49. [Crossref] [PubMed]
- Hsieh MS, Lin MW, Lee YH. Lung adenocarcinoma with sarcomatoid transformation after tyrosine kinase inhibitor treatment and chemotherapy. Lung Cancer 2019;137:76-84. [Crossref] [PubMed]
- Fallet V, Saffroy R, Girard N, et al. High-throughput somatic mutation profiling in pulmonary sarcomatoid carcinomas using the LungCarta™ Panel: exploring therapeutic targets. Ann Oncol 2015;26:1748-53. [Crossref] [PubMed]
- Schrock AB, Li SD, Frampton GM, et al. Pulmonary Sarcomatoid Carcinomas Commonly Harbor Either Potentially Targetable Genomic Alterations or High Tumor Mutational Burden as Observed by Comprehensive Genomic Profiling. J Thorac Oncol 2017;12:932-42. [Crossref] [PubMed]
- Alì G, Bruno R, Poma AM, et al. Whole transcriptome targeted gene quantification provides new insights on pulmonary sarcomatoid carcinomas. Sci Rep 2019;9:3536. [Crossref] [PubMed]
- Kaira K, Horie Y, Ayabe E, et al. Pulmonary pleomorphic carcinoma: a clinicopathological study including EGFR mutation analysis. J Thorac Oncol 2010;5:460-5. [Crossref] [PubMed]
- Ushiki A, Koizumi T, Kobayashi N, et al. Genetic heterogeneity of EGFR mutation in pleomorphic carcinoma of the lung: response to gefitinib and clinical outcome. Jpn J Clin Oncol 2009;39:267-70. [Crossref] [PubMed]
- Liu X, Jia Y, Shen Y, et al. Detection of frequent MET Exon 14 skipping events in pulmonary sarcomatoid carcinoma and response to targeted inhibition. J Clin Oncol 2015;33:8020. [Crossref]
- Kim EK, Kim KA, Lee CY, et al. Molecular Diagnostic Assays and Clinicopathologic Implications of MET Exon 14 Skipping Mutation in Non-small-cell Lung Cancer. Clin Lung Cancer 2019;20:e123-32. [Crossref] [PubMed]
- Han S, Fang J, Lu S, et al. Response and acquired resistance to savolitinib in a patient with pulmonary sarcomatoid carcinoma harboring MET exon 14 skipping mutation: a case report. Onco Targets Ther 2019;12:7323-8. [Crossref] [PubMed]
- Miranda O, Farooqui M, Siegfried JM. Status of Agents Targeting the HGF/c-Met Axis in Lung Cancer. Cancers (Basel) 2018;10:280. [Crossref] [PubMed]
- Paik PK, Felip E, Veillon R, et al. Tepotinib in Non-Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N Engl J Med 2020;383:931-43. [Crossref] [PubMed]
- Lu S, Fang J, Li X, et al. Phase II study of savolitinib in patients (pts) with pulmonary sarcomatoid carcinoma (PSC) and other types of non-small cell lung cancer (NSCLC) harboring MET exon 14 skipping mutations (METex14+). J Clin Oncol 2020;38:9519. [Crossref]
- O'Bryan JP. Pharmacological targeting of RAS: Recent success with direct inhibitors. Pharmacol Res 2019;139:503-11. [Crossref] [PubMed]
- Janes MR, Zhang J, Li LS, et al. Targeting KRAS Mutant Cancers with a Covalent G12C-Specific Inhibitor. Cell 2018;172:578-589.e17. [Crossref] [PubMed]
- Skoulidis F, Li BT, Dy GK, et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N Engl J Med 2021;384:2371-81. [Crossref] [PubMed]
- Sim JK, Chung SM, Choi JH, et al. Clinical and molecular characteristics of pulmonary sarcomatoid carcinoma. Korean J Intern Med 2018;33:737-44. [Crossref] [PubMed]
- Kim S, Kim MY, Koh J, et al. Programmed death-1 ligand 1 and 2 are highly expressed in pleomorphic carcinomas of the lung: Comparison of sarcomatous and carcinomatous areas. Eur J Cancer 2015;51:2698-707. [Crossref] [PubMed]
- Velcheti V, Rimm DL, Schalper KA. Sarcomatoid lung carcinomas show high levels of programmed death ligand-1 (PD-L1). J Thorac Oncol 2013;8:803-5. [Crossref] [PubMed]
- Chen P, Yu M, Zhang JL, et al. Significant benefits of pembrolizumab in treating refractory advanced pulmonary sarcomatoid carcinoma: A case report. World J Clin Cases 2020;8:2876-84. [Crossref] [PubMed]
- Cimpeanu E, Ahmed J, Zafar W, et al. Pembrolizumab - emerging treatment of pulmonary sarcomatoid carcinoma: A case report. World J Clin Cases 2020;8:97-102. [Crossref] [PubMed]
- Babacan NA, Pina IB, Signorelli D, et al. Relationship Between Programmed Death Receptor-Ligand 1 Expression and Response to Checkpoint Inhibitor Immunotherapy in Pulmonary Sarcomatoid Carcinoma: A Pooled Analysis. Clin Lung Cancer 2020;21:e456-63. [Crossref] [PubMed]
- Nishino K, Kunimasa K, Kimura M, et al. Favorable response to pembrolizumab after durvalumab failure in a stage III sarcomatoid carcinoma of the lung: a case report. BMC Pharmacol Toxicol 2020;21:26. [Crossref] [PubMed]
- Kakimoto T, Sasaki M, Yamamoto T, et al. A Histologically Complete Response to Immunotherapy Using Pembrolizumab in a Patient with Giant Cell Carcinoma of the Lung: An Additional Report and Literature Review. Case Rep Oncol Med 2019;2019:1763625. [Crossref] [PubMed]
- Domblides C, Leroy K, Monnet I, et al. Efficacy of Immune Checkpoint Inhibitors in Lung Sarcomatoid Carcinoma. J Thorac Oncol 2020;15:860-6. [Crossref] [PubMed]
- Jin C, Yang B. Dramatic Response of Pulmonary Sarcomatoid Carcinoma to Nivolumab Combined with Anlotinib: A Case Report. Case Rep Oncol 2020;13:601-5. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Filosso PL, Ruffini E, Asioli S, et al. Adenosquamous lung carcinomas: a histologic subtype with poor prognosis. Lung Cancer 2011;74:25-9. [Crossref] [PubMed]
- Cooke DT, Nguyen DV, Yang Y, et al. Survival comparison of adenosquamous, squamous cell, and adenocarcinoma of the lung after lobectomy. Ann Thorac Surg 2010;90:943-8. [Crossref] [PubMed]
- Maeda H, Matsumura A, Kawabata T, et al. Adenosquamous carcinoma of the lung: surgical results as compared with squamous cell and adenocarcinoma cases. Eur J Cardiothorac Surg 2012;41:357-61. [Crossref] [PubMed]
- Mordant P, Grand B, Cazes A, et al. Adenosquamous carcinoma of the lung: surgical management, pathologic characteristics, and prognostic implications. Ann Thorac Surg 2013;95:1189-95. [Crossref] [PubMed]
- Shiozawa T, Ishii G, Goto K, et al. Clinicopathological characteristics of EGFR mutated adenosquamous carcinoma of the lung. Pathol Int 2013;63:77-84. [Crossref] [PubMed]
- Watanabe Y, Tsuta K, Kusumoto M, et al. Clinicopathologic features and computed tomographic findings of 52 surgically resected adenosquamous carcinomas of the lung. Ann Thorac Surg 2014;97:245-51. [Crossref] [PubMed]
- Gawrychowski J, Bruliński K, Malinowski E, et al. Prognosis and survival after radical resection of primary adenosquamous lung carcinoma. Eur J Cardiothorac Surg 2005;27:686-92. [Crossref] [PubMed]
- Kawaguchi T, Takada M, Kubo A, et al. Performance status and smoking status are independent favorable prognostic factors for survival in non-small cell lung cancer: a comprehensive analysis of 26,957 patients with NSCLC. J Thorac Oncol 2010;5:620-30. [Crossref] [PubMed]
- Tsuhako K, Nakazato I, Hirayasu T, et al. Human papillomavirus DNA in adenosquamous carcinoma of the lung. J Clin Pathol 1998;51:741-9. [Crossref] [PubMed]
- Kanazawa H, Ebina M, Ino-Oka N, et al. Transition from squamous cell carcinoma to adenocarcinoma in adenosquamous carcinoma of the lung. Am J Pathol 2000;156:1289-98. [Crossref] [PubMed]
- Shi X, Wu H, Lu J, et al. Screening for major driver oncogene alterations in adenosquamous lung carcinoma using PCR coupled with next-generation and Sanger sequencing methods. Sci Rep 2016;6:22297. [Crossref] [PubMed]
- Lin G, Li C, Li PS, et al. Genomic origin and EGFR-TKI treatments of pulmonary adenosquamous carcinoma. Ann Oncol 2020;31:517-24. [Crossref] [PubMed]
- Liang J, Sui Q, Zheng Y, et al. A nomogram to predict prognosis of patients with lung adenosquamous carcinoma: a population-based study. J Thorac Dis 2020;12:2288-303. [Crossref] [PubMed]
- Wang J, Lian B, Ye L, et al. Clinicopathological characteristics and survival outcomes in adenosquamous carcinoma of the lung: a population-based study from the SEER database. Oncotarget 2017;9:8133-46. [Crossref] [PubMed]
- Hu M, Zhang B, Xu J, et al. Clinical Outcomes of Different Generations of EGFR Tyrosine Kinase Inhibitors in Advanced Lung Adenosquamous Carcinoma. Mol Diagn Ther 2019;23:773-9. [Crossref] [PubMed]
- Wang R, Pan Y, Li C, et al. Analysis of major known driver mutations and prognosis in resected adenosquamous lung carcinomas. J Thorac Oncol 2014;9:760-8. [Crossref] [PubMed]
- Vassella E, Langsch S, Dettmer MS, et al. Molecular profiling of lung adenosquamous carcinoma: hybrid or genuine type? Oncotarget 2015;6:23905-16. [Crossref] [PubMed]
- Liu Y, Dong Z, Jiang T, et al. Heterogeneity of PD-L1 Expression Among the Different Histological Components and Metastatic Lymph Nodes in Patients With Resected Lung Adenosquamous Carcinoma. Clin Lung Cancer 2018;19:e421-30. [Crossref] [PubMed]
- Shi X, Wu S, Sun J, et al. PD-L1 expression in lung adenosquamous carcinomas compared with the more common variants of non-small cell lung cancer. Sci Rep 2017;7:46209. [Crossref] [PubMed]
- Molina JR, Aubry MC, Lewis JE, et al. Primary salivary gland-type lung cancer: spectrum of clinical presentation, histopathologic and prognostic factors. Cancer 2007;110:2253-9. [Crossref] [PubMed]
- Bennett AK, Mills SE, Wick MR. Salivary-type neoplasms of the breast and lung. Semin Diagn Pathol 2003;20:279-304. [Crossref] [PubMed]
- Cho SH, Park SD, Ko TY, et al. Primary epithelial myoepithelial lung carcinoma. Korean J Thorac Cardiovasc Surg 2014;47:59-62. [Crossref] [PubMed]
- Chenevert J, Barnes LE, Chiosea SI. Mucoepidermoid carcinoma: a five-decade journey. Virchows Arch 2011;458:133-40. [Crossref] [PubMed]
- Zhu F, Liu Z, Hou Y, et al. Primary salivary gland-type lung cancer: clinicopathological analysis of 88 cases from China. J Thorac Oncol 2013;8:1578-84. [Crossref] [PubMed]
- Shen C, Wang X, Che G. A rare case of primary peripheral epithelial myoepithelial carcinoma of lung: Case report and literature review. Medicine (Baltimore) 2016;95:e4371. [Crossref] [PubMed]
- Goodwin CR, Khattab MH, Sankey EW, et al. Epithelial-myoepithelial carcinoma metastasis to the thoracic spine. J Clin Neurosci 2016;24:143-6. [Crossref] [PubMed]
- Flam JO, Brook CD, Sobel R, et al. Nasal epithelial myoepithelial carcinoma: An unusual cause of epiphora, a case report and review of the literature. Allergy Rhinol (Providence) 2015;6:133-7. [Crossref] [PubMed]
- Qin BD, Jiao XD, Liu K, et al. Clinical, pathological and treatment factors associated with the survival of patients with primary pulmonary salivary gland-type tumors. Lung Cancer 2018;126:174-81. [Crossref] [PubMed]
- Kumar V, Soni P, Garg M, et al. A Comparative Study of Primary Adenoid Cystic and Mucoepidermoid Carcinoma of Lung. Front Oncol 2018;8:153. [Crossref] [PubMed]
- Kang DY, Yoon YS, Kim HK, et al. Primary salivary gland-type lung cancer: surgical outcomes. Lung Cancer 2011;72:250-4. [Crossref] [PubMed]
- Hu MM, Hu Y, He JB, et al. Primary adenoid cystic carcinoma of the lung: Clinicopathological features, treatment and results. Oncol Lett 2015;9:1475-81. [Crossref] [PubMed]
- Sonobe S, Inoue K, Tachibana S, et al. A case of pulmonary mucoepidermoid carcinoma responding to carboplatin and paclitaxel. Jpn J Clin Oncol 2014;44:493-6. [Crossref] [PubMed]
- French C. NUT midline carcinoma. Nat Rev Cancer 2014;14:149-50. [Crossref] [PubMed]
- French CA. Pathogenesis of NUT midline carcinoma. Annu Rev Pathol 2012;7:247-65. [Crossref] [PubMed]
- French CA. The importance of diagnosing NUT midline carcinoma. Head Neck Pathol 2013;7:11-6. [Crossref] [PubMed]
- Reis-Filho JS, Torio B, Albergaria A, et al. p63 expression in normal skin and usual cutaneous carcinomas. J Cutan Pathol 2002;29:517-23. [Crossref] [PubMed]
- Harms A, Herpel E, Pfarr N, et al. NUT carcinoma of the thorax: Case report and review of the literature. Lung Cancer 2015;90:484-91. [Crossref] [PubMed]
- Mao N, Liao Z, Wu J, et al. Diagnosis of NUT carcinoma of lung origin by next-generation sequencing: case report and review of the literature. Cancer Biol Ther 2019;20:150-6. [Crossref] [PubMed]
- French CA, Miyoshi I, Kubonishi I, et al. BRD4-NUT fusion oncogene: a novel mechanism in aggressive carcinoma. Cancer Res 2003;63:304-7. [PubMed]
- Bauer DE, Mitchell CM, Strait KM, et al. Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clin Cancer Res 2012;18:5773-9. [Crossref] [PubMed]
- Chau NG, Mitchell CM, Aserlind A, et al. Aggressive treatment and survival outcomes in NUT midline carcinoma (NMC) of the head and neck (HN). J Clin Oncol 2014;32:6057. [Crossref]
- French CA, Ramirez CL, Kolmakova J, et al. BRD-NUT oncoproteins: a family of closely related nuclear proteins that block epithelial differentiation and maintain the growth of carcinoma cells. Oncogene 2008;27:2237-42. [Crossref] [PubMed]
- French CA, Rahman S, Walsh EM, et al. NSD3-NUT fusion oncoprotein in NUT midline carcinoma: implications for a novel oncogenic mechanism. Cancer Discov 2014;4:928-41. [Crossref] [PubMed]
- Giridhar P, Mallick S, Kashyap L, et al. Patterns of care and impact of prognostic factors in the outcome of NUT midline carcinoma: a systematic review and individual patient data analysis of 119 cases. Eur Arch Otorhinolaryngol 2018;275:815-21. [Crossref] [PubMed]
- French CA. NUT Carcinoma: Clinicopathologic features, pathogenesis, and treatment. Pathol Int 2018;68:583-95. [Crossref] [PubMed]
- Filippakopoulos P, Qi J, Picaud S, et al. Selective inhibition of BET bromodomains. Nature 2010;468:1067-73. [Crossref] [PubMed]
- Stathis A, Zucca E, Bekradda M, et al. Clinical Response of Carcinomas Harboring the BRD4-NUT Oncoprotein to the Targeted Bromodomain Inhibitor OTX015/MK-8628. Cancer Discov 2016;6:492-500. [Crossref] [PubMed]
- O’Dwyer PJ, Piha-Paul SA, French C, et al. Abstract CT014: GSK525762, a selective bromodomain (BRD) and extra terminal protein (BET) inhibitor: results from part 1 of a phase I/II open-label single-agent study in patients with NUT midline carcinoma (NMC) and other cancers. Cancer Res 2016;76:CT014. [Crossref]
- Maher OM, Christensen AM, Yedururi S, et al. Histone deacetylase inhibitor for NUT midline carcinoma. Pediatr Blood Cancer 2015;62:715-7. [Crossref] [PubMed]
- Morrison-Smith CD, Knox TM, Filic I, et al. Combined Targeting of the BRD4-NUT-p300 Axis in NUT Midline Carcinoma by Dual Selective Bromodomain Inhibitor, NEO2734. Mol Cancer Ther 2020;19:1406-14. [Crossref] [PubMed]
- Maur M, Toss A, Dominici M, et al. Impressive Response to Dose-Dense Chemotherapy in a Patient with NUT Midline Carcinoma. Am J Case Rep 2015;16:424-9. [Crossref] [PubMed]
- Arimizu K, Hirano G, Makiyama C, et al. NUT carcinoma of the nasal cavity that responded to a chemotherapy regimen for Ewing's sarcoma family of tumors: a case report. BMC Cancer 2018;18:1134. [Crossref] [PubMed]
- Sopfe J, Greffe B, Treece AL. Metastatic NUT Midline Carcinoma Treated With Aggressive Neoadjuvant Chemotherapy, Radiation, and Resection: A Case Report and Review of the Literature. J Pediatr Hematol Oncol 2021;43:e73-5. [Crossref] [PubMed]
- Mertens F, Wiebe T, Adlercreutz C, et al. Successful treatment of a child with t(15;19)-positive tumor. Pediatr Blood Cancer 2007;49:1015-7. [Crossref] [PubMed]
- Joel S, Weschenfelder F, Schleussner E, et al. NUT midline carcinoma in a young pregnant female: a case report. World J Surg Oncol 2020;18:290. [Crossref] [PubMed]
- Le Loarer F, Watson S, Pierron G, et al. SMARCA4 inactivation defines a group of undifferentiated thoracic malignancies transcriptionally related to BAF-deficient sarcomas. Nat Genet 2015;47:1200-5. [Crossref] [PubMed]
- Crombé A, Alberti N, Villard N, et al. Imaging features of SMARCA4-deficient thoracic sarcomas: a multi-centric study of 21 patients. Eur Radiol 2019;29:4730-41. [Crossref] [PubMed]
- Perret R, Chalabreysse L, Watson S, et al. SMARCA4-deficient Thoracic Sarcomas: Clinicopathologic Study of 30 Cases With an Emphasis on Their Nosology and Differential Diagnoses. Am J Surg Pathol 2019;43:455-65. [Crossref] [PubMed]
- Sauter JL, Graham RP, Larsen BT, et al. SMARCA4-deficient thoracic sarcoma: a distinctive clinicopathological entity with undifferentiated rhabdoid morphology and aggressive behavior. Mod Pathol 2017;30:1422-32. [Crossref] [PubMed]
- Yoshida A, Kobayashi E, Kubo T, et al. Clinicopathological and molecular characterization of SMARCA4-deficient thoracic sarcomas with comparison to potentially related entities. Mod Pathol 2017;30:797-809. [Crossref] [PubMed]
- Takeda M, Tani Y, Saijo N, et al. Cytopathological Features of SMARCA4-Deficient Thoracic Sarcoma: Report of 2 Cases and Review of the Literature. Int J Surg Pathol 2020;28:109-14. [Crossref] [PubMed]
- Rekhtman N, Montecalvo J, Chang JC, et al. SMARCA4-Deficient Thoracic Sarcomatoid Tumors Represent Primarily Smoking-Related Undifferentiated Carcinomas Rather Than Primary Thoracic Sarcomas. J Thorac Oncol 2020;15:231-47. [Crossref] [PubMed]
- Groisberg R, Hong DS, Behrang A, et al. Characteristics and outcomes of patients with advanced sarcoma enrolled in early phase immunotherapy trials. J Immunother Cancer 2017;5:100. [Crossref] [PubMed]
- Tawbi HA, Burgess M, Bolejack V, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol 2017;18:1493-501. [Crossref] [PubMed]
- Toulmonde M, Penel N, Adam J, et al. Use of PD-1 Targeting, Macrophage Infiltration, and IDO Pathway Activation in Sarcomas: A Phase 2 Clinical Trial. JAMA Oncol 2018;4:93-7. [Crossref] [PubMed]
- Blay JY, Penel N, Ray-Coquard IL, et al. High clinical activity of pembrolizumab in chordoma, alveolar soft part sarcoma (ASPS) and other rare sarcoma histotypes: The French AcSé pembrolizumab study from Unicancer. J Clin Oncol 2021;39:11520. [Crossref]
- Gantzer J, Davidson G, Vokshi B, et al. Immune-Desert Tumor Microenvironment in Thoracic SMARCA4-Deficient Undifferentiated Tumors with Limited Efficacy of Immune Checkpoint Inhibitors. Oncologist 2022;27:501-11. [Crossref] [PubMed]
- Dieu-Nosjean MC, Goc J, Giraldo NA, et al. Tertiary lymphoid structures in cancer and beyond. Trends Immunol 2014;35:571-80. [Crossref] [PubMed]
- Engelhard VH, Rodriguez AB, Mauldin IS, et al. Immune Cell Infiltration and Tertiary Lymphoid Structures as Determinants of Antitumor Immunity. J Immunol 2018;200:432-42. [Crossref] [PubMed]
- Sautès-Fridman C, Petitprez F, Calderaro J, et al. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer 2019;19:307-25. [Crossref] [PubMed]
- Henon C, Blay JY, Massard C, et al. Long lasting major response to pembrolizumab in a thoracic malignant rhabdoid-like SMARCA4-deficient tumor. Ann Oncol 2019;30:1401-3. [Crossref] [PubMed]
- Takada K, Sugita S, Murase K, et al. Exceptionally rapid response to pembrolizumab in a SMARCA4-deficient thoracic sarcoma overexpressing PD-L1: A case report. Thorac Cancer 2019;10:2312-5. [Crossref] [PubMed]
- Anžič N, Krasniqi F, Eberhardt AL, et al. Ipilimumab and Pembrolizumab Mixed Response in a 41-Year-Old Patient with SMARCA4-Deficient Thoracic Sarcoma: An Interdisciplinary Case Study. Case Rep Oncol 2021;14:706-15. [Crossref] [PubMed]
- Kawachi H, Kunimasa K, Kukita Y, et al. Atezolizumab with bevacizumab, paclitaxel and carboplatin was effective for patients with SMARCA4-deficient thoracic sarcoma. Immunotherapy 2021;13:799-806. [Crossref] [PubMed]
- Shorstova T, Marques M, Su J, et al. SWI/SNF-Compromised Cancers Are Susceptible to Bromodomain Inhibitors. Cancer Res 2019;79:2761-74. [Crossref] [PubMed]
- Chan-Penebre E, Armstrong K, Drew A, et al. Selective Killing of SMARCA2- and SMARCA4-deficient Small Cell Carcinoma of the Ovary, Hypercalcemic Type Cells by Inhibition of EZH2: In Vitro and In Vivo Preclinical Models. Mol Cancer Ther 2017;16:850-60. [Crossref] [PubMed]
Cite this article as: Pisano C, Witel G, De Filippis M, Listì A, Napoli F, Righi L, Novello S, Bironzo P. Moving through rare lung cancer histologies: a narrative review on diagnosis and treatment of selected infrequent entities. Precis Cancer Med 2022;5:27.

