
Giant perivascular spaces in brain: case report with a comprehensive literature review
Introduction
The perivascular spaces (PVSs) of the brain are small fluid-filled cystic spaces that surround the walls of vascular structures (arteries, arterioles, veins, and venules) along their course across the brain parenchyma. The term Virchow-Robin space was coined after the German pathologist Rudolf Virchow and the French anatomist Charles Philippe Robin who first identified and confirmed the existence of such spaces (1,2). The physiologic function of these spaces is not fully understood but they are considered equivalent to the systemic lymphatic pathways, which help in draining interstitial fluid from the extracellular spaces within the brain parenchyma (glymphatic system), including removal of solutes and clearing metabolic waste and may play an immunological role as well (3-5). With the advancements in imaging techniques, they have been noted to occur in nearly all individuals. Usually found bilaterally, they are not always symmetric (6,7). The structure, size, and histology of these spaces also vary based on the location and the surrounding vascular structures (artery vs. vein) (8,9). These PVSs are in direct communication with the subpial space and are present at specific locations of the brain. The PVSs are often associated with aging, vascular, and neurological conditions. Dysfunctional PVSs can sometimes grow in size and form giant PVSs (>15 mm) occurring in around 1.6% to 3% of the population (3,9). These giant PVSs, also known as tumefactive PVSs, cavernous dilatations, or Poirier’s type IIIb expanding lacunae, can often be mistaken on imaging for other vascular, neoplastic, infective, or immunologic lesions. This misdiagnosis can lead to unnecessary workup and treatment. Here, we discuss a case of giant PVS and also review the normal appearance of PVS and its differential diagnosis. We present the following case in accordance with the CARE reporting checklist (available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-3/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
A 70-year-old conscious, well-oriented female presented in the emergency department with a complaint of acute onset dizziness with difficulty in ambulation. She had intermittent vertigo for several years but was never investigated for the same. Clinical examination did not show any neurological deficit. Her medical history was positive for diabetes and hypertension. There was no relevant surgical history. A computed tomography (CT) scan of the head without intravenous contrast showed a circumscribed, lobulated, multiseptated cystic appearing lesion with cerebrospinal fluid (CSF) density in the right parietal subcortical region (Figure 1). In the absence of prior imaging, the differential diagnosis of cystic encephalomalacia or neoplasm and follow-up with contrast magnetic resonance imaging (MRI) of the brain was suggested. The patient was discharged after her symptoms improved with conservative management with meclizine. She then underwent MRI of the brain without and with contrast after 2 weeks, which showed a circumscribed, multiseptated, non-enhancing cystic lesion in right parietal subcortical white matter (Figures 2,3). There were a few other smaller but similar non-enhancing lesions adjacent to the larger structure. There was no associated mass effect or perilesional edema. The overlying cortex was unremarkable. The imaging findings were consistent with giant PVS and the possibility of neoplasm was discarded. Patient has no symptoms related to intracranial neoplasm and has been stable since then.

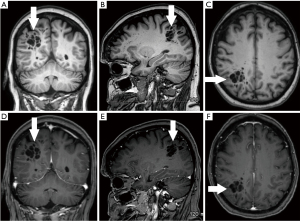
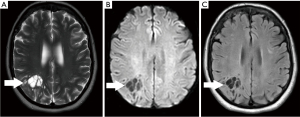
The article will also briefly discuss the review of literature which was performed in January 2022 using the PubMed/MEDLINE and Google Scholar search engines with the following search terms: Giant perivascular space, cystic neoplasm, complete recovery, dilated perivascular spaces. All original research articles, meta-analysis, case reports, and review articles were included from 1986 to 2022. All the studies that came up using the mentioned keywords were included. Exclusion criteria were as follows: (I) studies where the diagnoses was not clearly established; (II) studies in which imaging findings were not mentioned; and (III) studies published before 1986. Literature search was performed by SB and DG and was verified by all the authors.
Discussion
The PVSs are generally small (<2 mm) and can be seen in the MR images of all age groups and are typically asymptomatic (10). The detection rate of PVSs at the MRI is also influenced by the techniques used, viz., heavier T2-weighted imaging, thinner sections, higher-field strength resulting in better spatial resolution, contrast, and better visualization of VR spaces, thus leading to an increase in the prevalence of the same (9,11-16). The presence of enlarged PVSs (>5 mm) has been found and correlated with different physiological and pathologic neurological conditions such as aging, arteriosclerosis, hypertension, mild cognitive impairment (MCI), dementia, Alzheimer disease, and Parkinson’s disease (17-22). Around 2–3% of healthy individuals show enlarged PVSs in their MR images (19,20). The proposed mechanisms that may lead to the formation of dilated PVS are alterations in permeability of the wall of the vessels, perivascular demyelination, fibrosis/obstruction of lymphatic drainage pathways, or ex-vacuo dilatation secondary to brain atrophy (23-27). It also hypothesized that fenestrations in the pial layers that surround these vascular structures might lead to excessive collection of interstitial fluid in these spaces leading to the enlargement of the PVSs. This might explain the preferential occurrence of giant PVSs in the mesencephalo-thalamic region where the vasculature is surrounded by two layers of pia mater (27). Sometimes they get markedly dilated, almost resembling other cystic pathologies like infectious processes or, benign or malignant neoplasms. At times the PVS (as a solitary lesion or a group of multiple contiguous spaces) become fairly large and the ones that are 15 mm or larger are termed as giant/tumefactive PVSs (28,29).
There are three subtypes of dilated PVSs, classified according to their anatomical location. Type I PVS are the most common and found along the lenticulostriate arteries as they pass through the anterior-perforated substance to enter the basal ganglia. Type II lesions are located around perforating medullary arteries as they course from the cortical gray matter into the cerebral white matter. Type III lesions are seen in the mesencephalic region surrounding the penetrating branches of the collicular, thalamo-perforating, and other accessory collicular arteries (8-10,30-32). A new location for PVS has been recently described in the white matter of the anterior superior temporal lobe and is now recognized as the fourth category. Although PVSs are most commonly found around the lenticulostriate arteries (type I), the giant PVSs occur most commonly as type III lesions, located in the mesencephalo-thalamic region (30-32).
On MRI, the PVSs appear as sharply defined cystic structures with variable shapes (oval, round or tubular) that are more often found in groups than singly. The signal intensities of the PVS appear to be visually identical to those of CSF. However, on actual measurement, the signal intensities of the PVS are lower than the CSF-containing structures (8,9). This difference in signal intensity can be attributed to the fact that the fluid in PVS is entrapped and by partial volume effects as the fluid in the PVS with an in-house vessel is lesser than a similar volume cell. PVSs, being communicating compartments, show no restriction in diffusion on diffusion-weighted images. These spaces do not enhance with contrast material. The surrounding brain parenchyma usually exhibits normal signal intensity, especially in patients with small to moderate-sized spaces (2–5 mm) (33,34). However, PVSs that are found in white matter often show some perilesional hyperintensities, especially on T2 weighted images and on FLAIR sequence images. A study showed that abnormal white matter hyperintensities can be found in nearly 30% of the elderly population and some of these lesions are debated to be due to the presence of dilated PVSs (26,35,36). Occasionally, the PVS can become significantly big in size and develop a complex cystic structure that may put forth a variety of differentials like infectious and parasitic cysts, neuroepithelial cysts, cystic neoplasms, cystic lacunar infarctions, and deposition disorders like mucopolysaccharidosis (37-39). Salzman et al. (28) studied and reviewed 37 patients with giant VR spaces and reported that most often these appear as clusters of variably sized cysts in the mesencephalo-thalamic region and cerebral white matter. Their study showed that most patients present with headaches, however, patients may also present with other non-specific neurological symptoms including dizziness, loss of memory, visual problems, seizure, syncope, poor balance, and poor concentration. However, studies have shown that clinical features are not always correlated to the imaging findings, and many asymptomatic patients maybe incidentally found to have giant PVSs on imaging done for other indications (34,40,41). Giant PVSs in the mesencephalo-thalamic region may cause compression of the third ventricle or the Sylvian aqueduct leading to the development of hydrocephalus (29,42). Occasionally, even mildly dilated PVSs, which are not large enough to be labeled as giant/tumefactive PVS, may cause hydrocephalus (42). In addition to the classic appearance of multiple, variably sized, oval, circumscribed, CSF-filled cysts, PVSs are best distinguished from cystic neoplasms by lack of contrast enhancement and stability in appearance over time (23).
Dilated PVSs, especially the giant (tumefactive) ones can be confusing on the MR images and mimic a variety of different pathologies viz., infectious, vascular, congenital, and neoplastic processes with cystic appearances. Also, PVSs can act as a conduit for the spread of infections, inflammatory diseases, and neoplasms (23). Hence, it is important to recognize and comprehend the typical clinical and imaging appearances of these pathologies as well in order to suggest the correct diagnosis. The MRI characteristics of common differentials of dilated PVS are discussed.
Chronic vascular or inflammatory lesions can be confused with dilated PVS especially if they are seen in areas typical for PVS. Lacunar infarcts due to blockade of the corresponding penetrating arteries may cause confusion with a PVS in the basal ganglia, thalamus, pons, and periventricular white matter. The chronic infarcts are the ones that cause greater confusion with dilated PVS as they present with a central cystic area (encephalomalacia). However, they tend to be asymmetric, wedge-shaped lesions which are usually >5 mm and show a hyperintense rim on the T2/FLAIR images (43). Cystic periventricular leukomalacia and porencephalic cysts may occur due to prenatal or perinatal hypoxic/ischemic events and present as cavitary cystic lesions in the periventricular white matter and mimic PVSs (44). They usually show abnormal signals on the T2/FLAIR images with the porencephalic cysts communicating with the ventricular system/subarachnoid space. These features are helpful in differentiating them from dilated PVS. Finally, chronic multiple sclerosis presents with lesions in the white matter of the periventricular and juxtacortical region (45). The clinical presentation along with the perilesional hyperintense T2/FLAIR signal are generally helpful in distinguishing from PVS. In the acute stage, MS lesions are isointense or mildly hypointense to brain parenchyma on T1-weighted images with enhancement being dependent on the degree of inflammation (46).
Infectious lesions like neurocysticercosis, toxoplasmosis, cryptococcus can present as cystic lesions in the brain with cysticercosis being the most common and the other two occurring in immunocompromised individuals particularly those with HIV. Neurocysticercosis, initially show up as simple cysts at the junction of gray-white matter and basal ganglia region, looking like a dilated PVS, but as they progress through the different stages (colloidal, granular, and nodular) the lesion shows hyperintensity of the cyst content with rim enhancement and perilesional edema and finally the lesions appear shrunken with calcifications (47). Toxoplasmosis usually occurs within basal ganglia and corticomedullary junction showing the typical “target sign” with multiple peripherally or ring-enhancing lesions, sometimes with intrinsic T1 shortening (48). Cryptococcosis is caused by Cryptococcus neoformans (an opportunistic fungus) and usually starts as a meningeal infection with little inflammation, mainly due to the immunocompromised state of the host per se and the immunosuppressive effect of the organism’s capsule itself. The infection tends to spread along the PVS, distending the latter with the capsular mucoid material, leading to dilated PVS on the MRI and may enlarge to form gelatinous pseudocysts (49-51). The meningeal exudate or subsequent meningeal adhesions may lead to the formation of hydrocephalus. The hyperintense images of these lesions on T2-weighted MR and FLAIR help to differentiate them from normal PVS. Contrast enhancement is rarely seen (52). The mucoid/gelatinous nature of the cyst content restricts on the diffusion-weighted images.
Giant PVS can present with unusual conformations and mass effects and get mistaken for a benign or low-grade cystic neoplasm like pilocytic astrocytoma, dysembryoplastic neuroepithelial tumor (DNET), multinodular and vacuolating neuronal tumor (MVNT), ganglioglioma, or pleomorphic xanthoastrocytoma (28,38,39). The findings of solid components within the cystic areas with a possibility of enhancement with contrast, surrounding edema, and contents that usually are not isointense with CSF favor a neoplasm over PVS. The DNETs are slow growing WHO grade I benign tumors typically involving medial temporal lobes and presenting with complex partial seizures which on imaging usually have a “bubbly” cystic appearance with somewhat widespread T2/FLAIR signal abnormality and minimal contrast enhancement (53). MVNT is a mixed glial neuronal benign lesion presenting with seizures in nearly a third of the patients and on imaging are seen as a group of tiny cystic and nodular subcortical lesions that exhibit abnormal signals on T2/FLAIR images and rarely show contrast enhancement (54-56). Sometimes all the typical findings might not be seen and the differentiation between giant PVS and cystic brain tumors may become perplexing and a follow-up MRI may be of help to resolve the dilemma.
Benign cystic lesions like neuroglial cysts, arachnoid cysts, and neurenteric cysts can mimic dilated PVS. Neuroglial cysts are congenital epithelial lined lesions formed from the sequestration of the embryonic neural tube elements in the developing white matter and can be located at any site of the neuroaxis (57,58). Arachnoid cysts are extra-axial CSF-filled diverticular structures arising from the arachnoid membranes (59). Neurenteric cysts are congenital endodermal lesions commonly seen in the posterior fossa of the cranial cavity (60). Finally, choroidal cysts can arise from arachnoid or neuroepithelium and are seen at the level of the choroidal fissure (61). On imaging, these benign cysts share many features with dilated PVSs but can be easily differentiated from the latter by their location and usually solitary nature.
In conclusion, PVSs are normally occurring spaces found at specific locations and help in clearing the interstitial fluid in the brain. The presence of the PVSs may act as a biomarker to detect the presence of various vascular and neurological conditions. Dysfunctional PVSs might grow in size and become giant PVSs that can be confused with other potentially serious neurological conditions. While some patients may be asymptomatic and incidentally diagnosed, others can present with headaches, signs of increased intracranial pressure due to obstructive hydrocephalus, and other non-specific neurological symptoms. Knowledge of the location and characteristic appearance can help clinicians to correctly identify PVSs and differentiating it from its mimics on imaging to avoid any unnecessary workup or treatment.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-3/rc
Peer Review File: Available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-3/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-3/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Robin C. Recherches sur quelques particularites de la structure des capillaires de l’encephale. J Physiol Homme Anim 1859;2:537-48.
- Virchow R. Ueber die Erweiterung kleinerer Gefaesse. Archiv Pathol Anat Physiol Klin Med 1851;3:427-62. [Crossref]
- Zhang ET, Inman CB, Weller RO. Interrelationships of the pia mater and the perivascular (Virchow-Robin) spaces in the human cerebrum. J Anat 1990;170:111-23. [PubMed]
- Schley D, Carare-Nnadi R, Please CP, et al. Mechanisms to explain the reverse perivascular transport of solutes out of the brain. J Theor Biol 2006;238:962-74. [Crossref] [PubMed]
- Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 2012;4:147ra111. [Crossref] [PubMed]
- Piantino J, Boespflug EL, Schwartz DL, et al. Characterization of MR Imaging-Visible Perivascular Spaces in the White Matter of Healthy Adolescents at 3T. AJNR Am J Neuroradiol 2020;41:2139-45. [Crossref] [PubMed]
- Barisano G, Sheikh-Bahaei N, Law M, et al. Body mass index, time of day and genetics affect perivascular spaces in the white matter. J Cereb Blood Flow Metab 2021;41:1563-78. [Crossref] [PubMed]
- Oztürk MH, Aydingöz U. Comparison of MR signal intensities of cerebral perivascular (Virchow-Robin) and subarachnoid spaces. J Comput Assist Tomogr 2002;26:902-4. [Crossref] [PubMed]
- Heier LA, Bauer CJ, Schwartz L, et al. Large Virchow-Robin spaces: MR-clinical correlation. AJNR Am J Neuroradiol 1989;10:929-36. [PubMed]
- Kwee RM, Kwee TC. Virchow-Robin spaces at MR imaging. Radiographics 2007;27:1071-86. [Crossref] [PubMed]
- Saeki N, Sato M, Kubota M, et al. MR imaging of normal perivascular space expansion at midbrain. AJNR Am J Neuroradiol 2005;26:566-71. [PubMed]
- Song CJ, Kim JH, Kier EL, et al. MR imaging and histologic features of subinsular bright spots on T2-weighted MR images: Virchow-Robin spaces of the extreme capsule and insular cortex. Radiology 2000;214:671-7. [Crossref] [PubMed]
- Takahashi M, Uematsu H, Hatabu H. MR imaging at high magnetic fields. Eur J Radiol 2003;46:45-52. [Crossref] [PubMed]
- Uematsu H, Dougherty L, Takahashi M, et al. A direct comparison of signal behavior between 4.0 and 1.5 T: a phantom study. Eur J Radiol 2003;45:154-9. [Crossref] [PubMed]
- Sasaki M, Inoue T, Tohyama K, et al. High-field MRI of the central nervous system: current approaches to clinical and microscopic imaging. Magn Reson Med Sci 2003;2:133-9. [Crossref] [PubMed]
- Barisano G, Law M, Custer RM, et al. Perivascular Space Imaging at Ultrahigh Field MR Imaging. Magn Reson Imaging Clin N Am 2021;29:67-75. [Crossref] [PubMed]
- Davis PC, Mirra SS, Alazraki N. The brain in older persons with and without dementia: findings on MR, PET, and SPECT images. AJR Am J Roentgenol 1994;162:1267-78. [Crossref] [PubMed]
- Miyakawa T, Hattori E, Shikai I, et al. Histopathological changes of chronic alcoholism. Folia Psychiatr Neurol Jpn 1977;31:253-61. [PubMed]
- Poirier J, Derouesné C. Distinguishing lacunar infarcts from dilatations of the perivascular space. J Neurol 1998;245:813-4. [Crossref] [PubMed]
- Achiron A, Faibel M. Sandlike appearance of Virchow-Robin spaces in early multiple sclerosis: a novel neuroradiologic marker. AJNR Am J Neuroradiol 2002;23:376-80. [PubMed]
- Sepehrband F, Barisano G, Sheikh-Bahaei N, et al. Volumetric distribution of perivascular space in relation to mild cognitive impairment. Neurobiol Aging 2021;99:28-43. [Crossref] [PubMed]
- Donahue EK, Murdos A, Jakowec MW, et al. Global and Regional Changes in Perivascular Space in Idiopathic and Familial Parkinson’s Disease. Mov Disord 2021;36:1126-36. [Crossref] [PubMed]
- Wardlaw JM, Benveniste H, Nedergaard M, et al. Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Rev Neurol 2020;16:137-53. [Crossref] [PubMed]
- Benhaïem-Sigaux N, Gray F, Gherardi R, et al. Expanding cerebellar lacunes due to dilatation of the perivascular space associated with Binswanger’s subcortical arteriosclerotic encephalopathy. Stroke 1987;18:1087-92. [Crossref] [PubMed]
- Hugh W. Origin of lacunes. Lancet 1965;2:19-21.
- Awad IA, Johnson PC, Spetzler RF, et al. Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. II. Postmortem pathological correlations. Stroke 1986;17:1090-7. [Crossref] [PubMed]
- Adachi M, Hosoya T, Haku T, et al. Dilated Virchow-Robin spaces: MRI pathological study. Neuroradiology 1998;40:27-31. [Crossref] [PubMed]
- Salzman KL, Osborn AG, House P, et al. Giant tumefactive perivascular spaces. AJNR Am J Neuroradiol 2005;26:298-305. [PubMed]
- Kanamalla US, Calabrò F, Jinkins JR. Cavernous dilatation of mesencephalic Virchow-Robin spaces with obstructive hydrocephalus. Neuroradiology 2000;42:881-4. [Crossref] [PubMed]
- Lim AT, Chandra RV, Trost NM, et al. Large anterior temporal Virchow-Robin spaces: unique MR imaging features. Neuroradiology 2015;57:491-9. [Crossref] [PubMed]
- Rawal S, Croul SE, Willinsky RA, et al. Subcortical cystic lesions within the anterior superior temporal gyrus: a newly recognized characteristic location for dilated perivascular spaces. AJNR Am J Neuroradiol 2014;35:317-22. [Crossref] [PubMed]
- Rudie JD, Rauschecker AM, Nabavizadeh SA, et al. Neuroimaging of Dilated Perivascular Spaces: From Benign and Pathologic Causes to Mimics. J Neuroimaging 2018;28:139-49. [Crossref] [PubMed]
- Braffman BH, Zimmerman RA, Trojanowski JQ, et al. Brain MR: pathologic correlation with gross and histopathology. 1. Lacunar infarction and Virchow-Robin spaces. AJR Am J Roentgenol 1988;151:551-8. [Crossref] [PubMed]
- Demaerel P, Wilms G, Baert AL, et al. Widening of Virchow-Robin spaces. AJNR Am J Neuroradiol 1996;17:800-1. [PubMed]
- Gerard G, Weisberg LA. MRI periventricular lesions in adults. Neurology 1986;36:998-1001. [Crossref] [PubMed]
- Jungreis CA, Kanal E, Hirsch WL, et al. Normal perivascular spaces mimicking lacunar infarction: MR imaging. Radiology 1988;169:101-4. [Crossref] [PubMed]
- Cakirer S. MR imaging findings in tumefactive perivascular spaces. Acta Radiol 2003;44:673-4. [Crossref] [PubMed]
- Davis G, Fitt GJ, Kalnins RM, et al. Increased perivascular spaces mimicking frontal lobe tumor. J Neurosurg 2002;97:723. [Crossref] [PubMed]
- Romi F, Tysnes OB, Kråkenes J, et al. Cystic dilation of Virchow-Robin spaces in the midbrain. Eur Neurol 2002;47:186-8. [Crossref] [PubMed]
- Ogawa T, Okudera T, Fukasawa H, et al. Unusual widening of Virchow-Robin spaces: MR appearance. AJNR Am J Neuroradiol 1995;16:1238-42. [PubMed]
- Ugawa Y, Shirouzu I, Terao Y, et al. Physiological analyses of a patient with extreme widening of Virchow-Robin spaces. J Neurol Sci 1998;159:25-7. [Crossref] [PubMed]
- Papayannis CE, Saidon P, Rugilo CA, et al. Expanding Virchow Robin spaces in the midbrain causing hydrocephalus. AJNR Am J Neuroradiol 2003;24:1399-403. [PubMed]
- Bokura H, Kobayashi S, Yamaguchi S. Distinguishing silent lacunar infarction from enlarged Virchow-Robin spaces: a magnetic resonance imaging and pathological study. J Neurol 1998;245:116-22. [Crossref] [PubMed]
- Hinojosa-Rodríguez M, Harmony T, Carrillo-Prado C, et al. Clinical neuroimaging in the preterm infant: Diagnosis and prognosis. Neuroimage Clin 2017;16:355-68. [Crossref] [PubMed]
- Sarbu N, Shih RY, Jones RV, et al. White Matter Diseases with Radiologic-Pathologic Correlation. Radiographics 2016;36:1426-47. [Crossref] [PubMed]
- Pretorius PM, Quaghebeur G. The role of MRI in the diagnosis of MS. Clin Radiol 2003;58:434-48. [Crossref] [PubMed]
- Venkat B, Aggarwal N, Makhaik S, et al. A comprehensive review of imaging findings in human cysticercosis. Jpn J Radiol 2016;34:241-57. [Crossref] [PubMed]
- Masamed R, Meleis A, Lee EW, et al. Cerebral toxoplasmosis: case review and description of a new imaging sign. Clin Radiol 2009;64:560-3. [Crossref] [PubMed]
- Mathews VP, Alo PL, Glass JD, et al. AIDS-related CNS cryptococcosis: radiologic-pathologic correlation. AJNR Am J Neuroradiol 1992;13:1477-86. [PubMed]
- Tien RD, Chu PK, Hesselink JR, et al. Intracranial cryptococcosis in immunocompromised patients: CT and MR findings in 29 cases. AJNR Am J Neuroradiol 1991;12:283-9. [PubMed]
- Wehn SM, Heinz ER, Burger PC, et al. Dilated Virchow-Robin spaces in cryptococcal meningitis associated with AIDS: CT and MR findings. J Comput Assist Tomogr 1989;13:756-62. [Crossref] [PubMed]
- Miszkiel KA, Hall-Craggs MA, Miller RF, et al. The spectrum of MRI findings in CNS cryptococcosis in AIDS. Clin Radiol 1996;51:842-50. [Crossref] [PubMed]
- Fernandez C, Girard N, Paz Paredes A, et al. The usefulness of MR imaging in the diagnosis of dysembryoplastic neuroepithelial tumor in children: a study of 14 cases. AJNR Am J Neuroradiol 2003;24:829-34. [PubMed]
- Alsufayan R, Alcaide-Leon P, de Tilly LN, et al. Natural history of lesions with the MR imaging appearance of multinodular and vacuolating neuronal tumor. Neuroradiology 2017;59:873-83. [Crossref] [PubMed]
- Huse JT, Edgar M, Halliday J, et al. Multinodular and vacuolating neuronal tumors of the cerebrum: 10 cases of a distinctive seizure-associated lesion. Brain Pathol 2013;23:515-24. [Crossref] [PubMed]
- Nunes RH, Hsu CC, da Rocha AJ, et al. Multinodular and Vacuolating Neuronal Tumor of the Cerebrum: A New “Leave Me Alone” Lesion with a Characteristic Imaging Pattern. AJNR Am J Neuroradiol 2017;38:1899-904. [Crossref] [PubMed]
- Epelman M, Daneman A, Blaser SI, et al. Differential diagnosis of intracranial cystic lesions at head US: correlation with CT and MR imaging. Radiographics 2006;26:173-96. [Crossref] [PubMed]
- Osborn AG, Preece MT. Intracranial cysts: radiologic-pathologic correlation and imaging approach. Radiology 2006;239:650-64. [Crossref] [PubMed]
- Al-Holou WN, Terman S, Kilburg C, et al. Prevalence and natural history of arachnoid cysts in adults. J Neurosurg 2013;118:222-31. [Crossref] [PubMed]
- Preece MT, Osborn AG, Chin SS, et al. Intracranial neurenteric cysts: imaging and pathology spectrum. AJNR Am J Neuroradiol 2006;27:1211-6. [PubMed]
- de Jong L, Thewissen L, van Loon J, et al. Choroidal fissure cerebrospinal fluid-containing cysts: case series, anatomical consideration, and review of the literature. World Neurosurg 2011;75:704-8. [Crossref] [PubMed]
Cite this article as: Cheraya G, Bajaj S, Sharma S, Gandhi D, Shah J, Parikh N, Gupta N. Giant perivascular spaces in brain: case report with a comprehensive literature review. Precis Cancer Med 2022;5:29.

