
Prospective and single-blinded evaluation of the multi-cancer Carcimun-test
Introduction
Timely detection of malignancy by early diagnosis and cancer screening provides patients with the opportunity to effectively benefit from cancer therapy because treatment response and, accordingly, survival gains are by far more pronounced at early stages of cancer (1). Decreased mortality rates of some cancers such as colorectal and breast cancer can at least be partially attributed to the establishment of corresponding cancer screening tests (2).
However, the vast majority of cancer patients is currently being diagnosed after the onset of symptoms at advanced stages when tumors have potentially progressed to a point where treatment options are limited. Diagnostic tools for screening only exist for a small subset of cancers and include microscopic analysis combined with DNA testing [cervical cancer (3)], skin exams (4), imaging techniques [breast cancer mammography (5), computed tomography (CT) colonography and colonoscopy (6) chest low-dose CT (7)], blood based tests [e.g. prostate-specific antigen (8) carcinoembryonic antigen (9)], and developing methods such as liquid biopsy [detection of circulating tumor cells and tumor derived DNA (10,11)]. However, many of these tests are limited by rather low sensitivity and specificity that range from 70% to 80% and approximately 60% to 70%, respectively (12). And in addition, sensitivity depends on tumor size, the patient’s age, disease history, and tissue composition (13-15).
Presently, most of these cancer screening strategies focus on specific tumor entities, contradicting the ‘paradox’ of cancer epidemiology, which says that the risk for any cancer over the course of the lifespan is generally high, while the risk for one specific cancer at a certain time is rather low (12). A more universal test for early diagnosis detecting the most frequent tumor entities would therefore be of highest significance for cancer management if test performance in terms of high sensitivity, specificity, and accuracy as well as applicability and accessibility are clinically adequate (16).
Most of the available in vitro diagnostic tests for early diagnosis of cancer base on the detection of tumor derived proteins or mutations and other tumor-inherent changes displaying malignancy. In contrast, the Carcimun-test (invented by Berthold Zwerger) presented in this publication bases on the observation that the optical absorption spectra of human blood plasma at a given wavelength may possess diagnostic significance (17) due to detectable and specific changes in pathological conditions (18), thereby offering an opportunity for the identification of cancer (19,20). In the Carcimun-test, plasma samples are processed and measured following an empirically developed and well standardized protocol to reveal shifted absorption maxima correlating with malignancy. However, the molecular mechanism to induce the underlying phenomena remains to be elucidated. Interestingly in this regard, in unpublished pilot studies we found that Carcimun-test results not only correlate with malignancy but also with acute and chronic inflammation, suggesting an involvement of the humoral immune system (21). Yet data from these pilot studies suggest that extinction values above a defined threshold indicate malignancy in individual patients if acute inflammation can be excluded.
In the long term, we believe that the clinical usage of the Carcimun-test might be possible in two scenarios. First, we hypothesize that the test can be applied as screening tool in asymptomatic individuals. Second, the test may be feasible to early diagnose cancer with meaningful accuracy after the occurrence of initial symptoms and unclear clinical signs. To further validate the second hypothesis, we conducted a prospective, single-blinded evaluation, including a 5-year follow-up on all-cause mortality in patients with a histologically proven malignant disease and healthy volunteers. We present the following article in accordance with the STARD reporting checklist (available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-35/rc).
Methods
Study design
This prospective, single-center, single-blinded study was conducted at the Medical University of Vienna, Austria, between April 2015 and April 2016, followed by a 5-year follow-up analysis. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Ethics Committee of Medical University of Vienna (No. EK 1651/2015, Medical University of Vienna, Austria) and informed consent was taken from all the patients.
Participants
Study participants were recruited as a convenience sample at the department of general surgery of the Medical University of Vienna, Austria. According to anamnestic information and histological examinations (i.e., patients who had been subjected to surgical treatment of cancer), participants were assigned to the group of cancer patients or healthy volunteers. To be included, participants were required to provide written informed consent. Primary exclusion criteria were the presence of acute and chronic inflammation, fever, autoimmune disease, the perception of being unhealthy, as well as an examination with contrast medium and isotopes and/or any therapeutic intervention in the 14 days before Carcimun-testing. If there was uncertainty as to the fulfillment of primary exclusion criteria, participants were tested for leukocytosis and increased levels of c-reactive protein and fibrinogen (secondary exclusion criteria).
The results of an unpublished pilot study of B. Zwerger and J. Groth has shown an accuracy of the “Zwerger-Test” (meanwhile named Carcimun-test) of about 89% (specificity as well as a sensitivity). Our null hypothesis was that the “Zwerger-Test” will not be less than 89% sensitive and 89% specific for detecting samples with malignant disease in the study cohort. A sample size calculation assuming an accuracy of 89% at a confidence interval of 5% revealed that a total cohort size of n=302 is appropriate. This estimation is valid for a fraction of positive (malignant) samples of 50% in the study cohort. All calculations were performed according to the formula of Jones et al. (22).
Carcimun-testing and follow-up procedures
For Carcimun-testing, one blood sample (9 mL S-Monovette K3E) per participant was obtained from the cubital vein and sampled in K3-EDTA tubes for plasma preparation and 3.2% buffered sodium citrate for standard coagulation analysis. Plasma was immediately separated by centrifugation (3,000 rpm, 5 minutes, room temperature) and transferred to tubes without additives. All plasma samples were blinded before testing. Details of the following Carcimun-test procedure cannot be given due to the ongoing patenting process. In brief, in accordance with a standardized protocol, conformational changes of specific plasma proteins were subjected to a defined biochemical process, and optically assessed using a commercial clinical chemistry analyzer, Thermo Scientific Konelab PRIME 60. Samples were analyzed in duplicates, and measurements were given as milli extinction units. A predefined cut-off value of 120 then differentiated between healthy individuals (£120) and cancer patients (>120). After the follow-up, the occurrence of subsequent cancer in each healthy volunteer was evaluated by a telephone interview, or, if not applicable, by examination of the latest available medical record. The Austrian death registry was further screened to detect fatal outcomes (all-cause mortality).
Statistical analysis
Normally distributed demographic data (age) were compared by the t-test for unpaired samples and presented as mean ± standard error of mean (SEM). Group differences in sex ratio and smoking status were tested by the by chi-square test. Crosstabs were used to analyze test performance (specificity, sensitivity, accuracy, and positive and negative predictive values). Based on actual test performance (rate of true positive, true negative, false positive and false negative) we calculated the contingence quotient using crosstabs. Post-hoc power calculation was conducted using the sample size of n=307 and a significance level α of 0.05. To assess the influence of tumor stage on the extinction level, the extinction data per stage category were averaged and stratified according to tumor stage in four tumor entities (breast, pancreas, colorectal, anus). Analysis was performed as univariate logistic regression. Data of this subgroup analysis are presented as mean (without group comparisons) since the numbers of cases per category were low. Survival curves were calculated by Kaplan-Meier estimation. All statistical analyses were performed with SPSS 26 (IBM Corp., USA).
Results
Participants characteristics
Out of 343 study participants screened, 16 (4.66%) and 20 (5.83%) participants were excluded due to due to fulfillment of primary and secondary exclusion criteria, respectively. The remaining 307 study participants, who all underwent the Carcimun-test at the beginning of the study, comprised 137 healthy volunteers and 170 cancer patients with proven malignancy (Figure 1). The mean follow-up period was 29.1±1.24 (SEM) months (range, 0–61.9 months). Mean age, sex ratio, and smoking status are summarized in Table 1. Malignancy in cancer patients was either proven by biopsy before blood withdrawal or thereafter by surgical removal of a lesion.
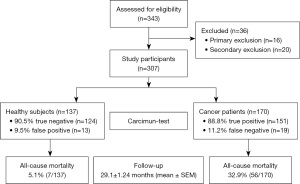
Table 1
| Participants’ characteristics | Healthy subjects (n=137) | Cancer patients§ (n=170) | Significance |
|---|---|---|---|
| Age (years, mean ± SEM) | 48.2±1.35 | 62.4±1.02 | P<0.004 |
| Sex (female/male) | 47/90 | 84/86 | P<0.008 |
| Smoking (yes/no) | 35/102 | 46/124 | NS |
§, tumor entities in cancer patients (number): secondary liver (n=28), breast (n=27), pancreas (n=21), colorectal (n=18), primary liver (n=13), neuroendocrine tumor (n=11), bile duct/Gallbladder (n=10), anus (n=10), esophageal (n=7), peritoneum (n=6), stomach (n=6), ovary (n=3), melanoma (n=3), sarcoma (n=3), lymphoma (n=2), skin (n=1), kidney (n=1). SEM, standard error of mean; NS, not significant.
Overall differences in test results
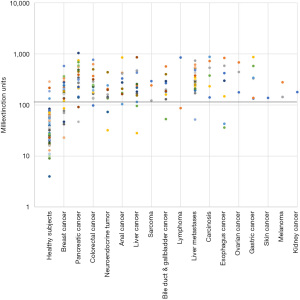
The mean extinction values were significantly higher in cancer patients than in healthy individuals (Table 2). Mean extinction values of cancers patients were 4.4 and 6.7-fold increased, when considering the entirety of study participants and the subgroup of correctly identified individuals, respectively. Moreover, extinction values of cancer patients including false negatives tended to positively correlate with tumor stage in breast, colorectal, pancreas, and anal cancer (Table 3). No optically visible changes to the sample indicating, e.g., massive protein precipitation were observed.
Table 2
| Study sample type | Healthy subjects (n=137) | Cancer patients (n=170) | Significance |
|---|---|---|---|
| All samples (mean extinction ± SEM) | 67.1±6.8 | 292.6±15.6 | P<0.000 |
| True negative/positive (mean extinction ± SEM) | 48.3±4.0 | 322.1±16.1 | P<0.000 |
SEM, standard error of mean.
Table 3
| Cancer type | Stage I | Stage II | Stage III | Stage IV |
|---|---|---|---|---|
| Breast cancer (mean extinction§) | 168 | 140 | 265 | 351 |
| Colorectal cancer (mean extinction§) | 136 | 235 | 253 | 338 |
| Pancreas cancer (mean extinction§) | – | 343 | 476 | 289 |
| Anal cancer (mean extinction§) | 242 | 156 | 371 | 566 |
§, data are presented as mean without standard error of mean due to low sample size in each category.
Identification of cancer patients by Carcimun-testing
On the basis of smaller pilot studies, a cut-off extinction value of 120 had been defined to differentiate between healthy individuals (£120) and cancer patients (>120). Applying this cut-off value, the Carcimun-test correctly identified 89.6% of study participants (275/307) as healthy individuals (124/137) and patients with different cancers (151/170). These data compare well to the previous unpublished pilot study results. False positive and false negative test results were observed in 9.5% (13) of healthy individuals and 11.2% (19) of cancer patients, respectively (Figures 1,2). In more detail, false negative results occurred in patients with breast cancer stage I (4 out of 12), II (2 out of 9), III (1 out of 4), IV (0 out of 2), pancreatic cancer stage II (0 out of 1), stage III (1 out of 9) and IV (1 out of 11), esophageal cancer stage II and IV; n=1 each, hepatoma (n=2), bile duct cancer patients (n=3), neuroendocrine tumor patients (n=2), colorectal cancer patient stage I (0 out of 2), stage II (0 out of 3), stage III (0 out of 5) and stage IV (1 out of 8), liver metastasis (n=1), and lymphoma (n=1).
Carcimun-test performance
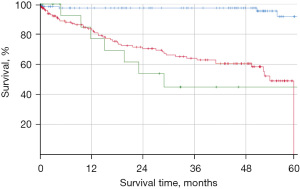
In our study, the Carcimun-test achieved high levels of accuracy (90.0%), sensitivity (88.8%), and specificity (91.2%). The positive and negative predictive values were 92.0% and 87.0%, respectively. Post-hoc power calculation revealed a power of 1.0 and thus a likelihood of 0% for a type 2 error. To evaluate the clinical relevance of the Carcimun-test results, we assessed the 5-year all-cause mortality of study participants who had correctly been identified as healthy individuals or cancer patients (Figure 3). All-cause mortality after 5 years was below 10% in healthy individuals and over 50% in cancer patients (P<0.002). The similarity of the survival curves for cancer patients tested either positive (true positive) or negative (false negative) suggests that the test indeed missed clinically relevant cancers (Figure 3). While no adverse events were observed by the blood withdrawal for the Carcimun-test, the reference standard was part of the standard work up of the patients and associated with the commonly known side effects.
Discussion
In this study, we assessed feasibility and performance of the novel Carcimun-test in cancer patients and healthy volunteers. The Carcimun-test demonstrated to be easily applicable in clinical practice and successfully differentiated between healthy subjects and cancer patients with high accuracy, sensitivity, and specificity. Carcimun-test results further correlated with long-term survival, thereby supporting the clinical relevance of Carcimun-test.
The activation of oncogenes and loss of tumor suppressors during cancerogenesis not only directly determines the characteristics of cancer cells, but also profoundly influences tumor-host interactions, involving the activation or inhibition of the immune system as well as the mutual relationship between the systemic and local immune milieu and metastasis (23). The exact mechanism of the Carcimun-test is unknown at this stage. However, we believe that in contrast to conventional tumor markers and screening methods that assess the presence of specific tumor proteins and mutations, the Carcimun-test detects the response of the host's cancer defense mechanisms detectable in human plasma. These mechanisms presumably involve the interplay of tumor cells with the host’s immune system. This might lead to subtle modifications of plasmatic proteins of the immune system. These modifications might be the subject of the Carcimun test principle leading to changes in absorption at given wavelength (24,25). To provide proof for this assumption research is currently conducted considering the following observation and two scenarios as potential explanation. As reported in this manuscript, we observed that the absorption measurements of the Carcimun test revealed a strong correlation with the disease stage. In the first and most frequent scenario, we would explain a modulation in absorption by protein precipitation and/or solubilisation processes. Obviously, precipitation/solubilisation phenomena change the amount of scattering and absorbing molecule mass, which will have a direct impact on the absorption. Given the pronounced changes in absorption we detected, we should expect a significant, and optically visible, amount of protein precipitation or solubilisation with increasing tumor stage. However, this was not the case. Therefore, we can reject that precipitation phenomena are the dominant molecular principle underlying the observed absorption changes. In a second scenario, the observed changes in absorption at a defined wavelength result from a shift of several absorption maxima which merge into the wavelength, at which the absorption measurement is conducted. This second scenario is consistent with our observations, i.e., no precipitation accompanying the increase in absorption, but raises the question towards underlying molecular phenomena that can induce such shifts in absorption maxima. Generally, aromatic groups such as tryptophan, tyrosine or phenylalanine as well as aromatic cofactors dominate the absorption behaviour of biomolecular samples. Each of these groups has a characteristic absorption maximum, which is frequently modulated by its environment (24,25).
As a consequence of this suspected more general underlying phenomenon, the Carcimun-test as we demonstrated in this manuscript is capable to detect a wide range of tumor entities, representing a major advantage over conventional methods for cancer screening and early diagnosis. Another advantage is that the Carcimun-test provides a methodically robust approach to detect cancer, thus enabling its clinical usage. Data presented in this article further indicate high levels of sensitivity (88.8%) and specificity (91.2%), corresponding to and even exceeding those of many screening tests (12), for example the carbohydrate antigen 19-9 test for pancreatic cancer [sensitivity: 70–90%; specificity: 68–91% (26)], the contrast enhanced spectral mammography for breast cancer [sensitivity: 85%, specificity: 77% (27)], and the fecal immunochemical test for colorectal cancer [sensitivity: 79%, specificity: 94% (28)]. However, the number of false positive and false negative test results of the Carcimun-test give raise to some concern. False positive test results misclassifying healthy subjects as having the disease would cause severe psychological consequences as well as unnecessary and possibly invasive diagnostic or therapeutic procedures. False negative test results misclassifying subjects with cancer as not having the disease would give them a false sense of security and potentially keep them from using additional diagnostic test, resulting in delayed diagnosis, and, in cases for which early therapy offers better chances for recovery, an increased risk of morbidity and mortality. To minimize those restrictions, we aim at further improving the test performance once the molecular mechanism of the test is fully understood. In addition, Carcimun-testing on a regular basis (e.g., annually) would reduce the risk of false test results, especially because our findings suggested a positive correlation of Carcimun-test mean extinction with tumor stage in breast, colorectal, pancreas, and anal cancer. At the current point in time, however, we consider the Carcimun-test as a complement rather than a substitute to other diagnostic tools.
A major disadvantage of the Carcimun-test might be the requirement to exclude inflammation in study participants. As demonstrated by the relatively low number of individuals excluded from this study due to the presence of inflammatory signs, this disadvantage might be negligible if the Carcimun-test is applied for early detection of cancer in single patients after the occurrence of first symptoms and clinical signs. Obviously, this disadvantage is more significant for its application as screening tool in asymptomatic individuals, but additional research is underway to further improve the Carcimun-test performance in this scenario.
In our study, the significant differences in the demographic characteristics of both study groups might limit the generalizability of results. However, in this study, we aimed at evaluating the clinical feasibility and general performance of our test. In addition to the basic research mentioned above, we will therefore conduct larger scale and properly designed studies to assess the performance of the Carcimun-test in both the diagnostic and the screening scenario.
Conclusions
The majority of tumor markers and diagnostic approaches presently available for the screening and early detection of cancer can only identify the presence of specific tumor types. Moreover, sensitivity and specificity of many cancer screening strategies are unsatisfactory, and clinical feasibility of some methodological approaches is limited. With the Carcimun-test, we present a novel, clinically feasible method to detect a broad range of tumor types, with high specificity, sensitivity, and accuracy. For now, we consider the Carcimun-test as a potential complement to existing diagnostic tools, rather than a substitute. Further research will be required to delineate the molecular mechanism of the Carcimun-test and to demonstrate the clinical relevance of the Carcimun-test for the screening and early diagnosis of cancer.
Acknowledgments
Funding: This study was privately funded by Berthold von und zu Zwerger.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-35/rc
Data Sharing Statement: Available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-35/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-35/coif). WV serves as an unpaid editorial board member of Precision Cancer Medicine from June 2020 to May 2022. WV received consulting fees from Siemens Healthineers for non-study related projects. BZ reports that this study was privately funded by himself. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Ethics Committee of Medical University of Vienna (EK 1651/2015, Medical University of Vienna, Austria) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Byers T, Wender RC, Jemal A, et al. The American Cancer Society challenge goal to reduce US cancer mortality by 50% between 1990 and 2015: Results and reflections. CA Cancer J Clin 2016;66:359-69. [Crossref] [PubMed]
- Tsikouras P, Zervoudis S, Manav B, et al. Cervical cancer: screening, diagnosis and staging. J BUON 2016;21:320-5. [PubMed]
- Wolff T, Tai E, Miller T. Screening for skin cancer: an update of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2009;150:194-8. [Crossref] [PubMed]
- van den Biggelaar FJ, Nelemans PJ, Flobbe K. Performance of radiographers in mammogram interpretation: a systematic review. Breast 2008;17:85-90. [Crossref] [PubMed]
- Pickhardt PJ, Hassan C, Halligan S, et al. Colorectal cancer: CT colonography and colonoscopy for detection--systematic review and meta-analysis. Radiology 2011;259:393-405. [Crossref] [PubMed]
- Hoffman RM, Sanchez R. Lung Cancer Screening. Med Clin North Am 2017;101:769-85. [Crossref] [PubMed]
- Brawer MK. Prostate-specific antigen. Semin Surg Oncol 2000;18:3-9. [Crossref] [PubMed]
- Young GP, Pedersen SK, Mansfield S, et al. A cross-sectional study comparing a blood test for methylated BCAT1 and IKZF1 tumor-derived DNA with CEA for detection of recurrent colorectal cancer. Cancer Med 2016;5:2763-72. [Crossref] [PubMed]
- Li J, Guan X, Fan Z, et al. Non-Invasive Biomarkers for Early Detection of Breast Cancer. Cancers (Basel) 2020;12:2767. [Crossref] [PubMed]
- Liu MC, Oxnard GR, Klein EA, et al. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann Oncol 2020;31:745-59. [Crossref] [PubMed]
- Schiffman JD, Fisher PG, Gibbs P. Early detection of cancer: past, present, and future. Am Soc Clin Oncol Educ Book 2015;57-65. [Crossref] [PubMed]
- Humphrey LL, Deffebach M, Pappas M, et al. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive services task force recommendation. Ann Intern Med 2013;159:411-20. [Crossref] [PubMed]
- Ohuchi N, Suzuki A, Sobue T, et al. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-cancer Randomized Trial (J-START): a randomised controlled trial. Lancet 2016;387:341-8. [Crossref] [PubMed]
- Sprague BL, Arao RF, Miglioretti DL, et al. National Performance Benchmarks for Modern Diagnostic Digital Mammography: Update from the Breast Cancer Surveillance Consortium. Radiology 2017;283:59-69. [Crossref] [PubMed]
- Shapley M, Mansell G, Jordan JL, et al. Positive predictive values of ≥5% in primary care for cancer: systematic review. Br J Gen Pract 2010;60:e366-77. [Crossref] [PubMed]
- Meinke M, Müller G, Helfmann J, et al. Optical properties of platelets and blood plasma and their influence on the optical behavior of whole blood in the visible to near infrared wavelength range. J Biomed Opt 2007;12:014024. [Crossref] [PubMed]
- Bosschaart N, Kok JH, Newsum AM, et al. Limitations and opportunities of transcutaneous bilirubin measurements. Pediatrics 2012;129:689-94. [Crossref] [PubMed]
- Franklin RG, Sanigar EB, Allen AJ. Spectrographic Studies of Cancer and Normal Blood Plasma: II. Absorption Spectra of Fractionated Cancer and Normal Human Blood Plasma. The American Journal of Cancer 1936;27:301-7. [Crossref]
- Lawaetz AJ, Bro R, Kamstrup-Nielsen M, et al. Fluorescence spectroscopy as a potential metabonomic tool for early detection of colorectal cancer. Metabolomics 2012;8:111-21. [Crossref]
- Adam JK, Odhav B, Bhoola KD. Immune responses in cancer. Pharmacol Ther 2003;99:113-32. [Crossref] [PubMed]
- Jones SR, Carley S, Harrison M. An introduction to power and sample size estimation. Emerg Med J 2003;20:453-8. [Crossref] [PubMed]
- Wellenstein MD, de Visser KE. Cancer-Cell-Intrinsic Mechanisms Shaping the Tumor Immune Landscape. Immunity 2018;48:399-416. [Crossref] [PubMed]
- Fiallo MM, Kozlowski H, Garnier-Suillerot A. Mitomycin antitumor compounds. Part 1. CD studies on their molecular structure. Eur J Pharm Sci 2001;12:487-94. [Crossref] [PubMed]
- Kemp W. Ultraviolet and Visible Spectroscopy. Organic Spectroscopy. London: Macmillan Education UK, 1991:243-83.
- Zhou B, Xu JW, Cheng YG, et al. Early detection of pancreatic cancer: Where are we now and where are we going? Int J Cancer 2017;141:231-41. [Crossref] [PubMed]
- Suter MB, Pesapane F, Agazzi GM, et al. Diagnostic accuracy of contrast-enhanced spectral mammography for breast lesions: A systematic review and meta-analysis. Breast 2020;53:8-17. [Crossref] [PubMed]
- Lee JK, Liles EG, Bent S, et al. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med 2014;160:171. [Crossref] [PubMed]
Cite this article as: Salat A, Voigt W, von und zu Zwerger B. Prospective and single-blinded evaluation of the multi-cancer Carcimun-test. Precis Cancer Med 2022;5:12.

