
RET-mutated non-small cell lung cancer treated with pralsetinib: a case series
Introduction
The advent of targeted therapies has radically changed the treatment landscape of non-small cell lung cancer (NSCLC) with oncogenic driver alterations (1,2). Several studies showed improved quality of like and significant survival benefit compared to chemotherapy. Thus, tyrosine kinase inhibitors (TKI) have become the standard of care for patients harbouring actionable mutations on EGFR, ALK, BRAF and ROS-1 (3-6).
Novel molecular diagnostics tools such as the next generation sequencing (NGS) have contributed to the discovery of further oncogenic drivers. Fusion of REarranged during Transfection (RET), HER2, c-MET exon 14 skipping mutation, KRASG12C mutation and NTRK have been identified as additional molecular targets in NSCLC patients (7-11).
RET gene rearrangements occur in 1–2% of lung adenocarcinomas, especially in young and non-smoking patients (12-14). Pralsetinib is an oral TKI with potent anti-RET activity. A multicenter and multi-cohort trial showed overall responses in 61% of patients previously treated with platinum-based chemotherapy, and in 70% of treatment-naive patients (15). Based on these results, Food and Drug Administration (FDA) granted accelerated approval to pralsetinib for metastatic RET fusion-positive NSCLC in September 2020. Panel and international guidelines recommends pralsetinib as a first-line or subsequent therapy option for patients with metastatic NSCLC who are positive for RET rearrangements (16). Additionally, the efficacy of selpercatinib, an ATP-competitive, highly selective small-molecule inhibitor of RET kinase, was also demonstrated in both pre-treated and naïve patient, with an objective response of 64% and 85% respectively (17).
In Italy, the compassionate use of pralsetinib allowed to target pretreated NSCLC patients harboring RET fusions. Ongoing trials are evaluating pralsetinib as front-line treatment for metastatic or unresectable locally advanced disease (18,19). As described in a recent report, pralsetinib was also successfully ad neoadjuvant therapy (20).
Due to rarity of RET fusion and novelty of these target therapies, observational and “real world” evidence are missing. We report four clinical cases of RET-mutated NSCLC patients treated with pralsetinib. To our knowledge this is the first clinical series on metastatic RET positive NSCLC treated with pralsetinib.
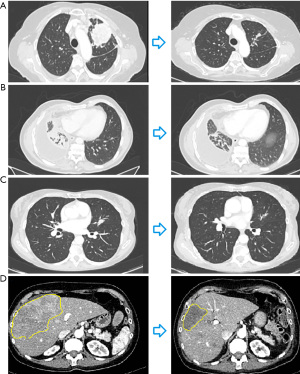
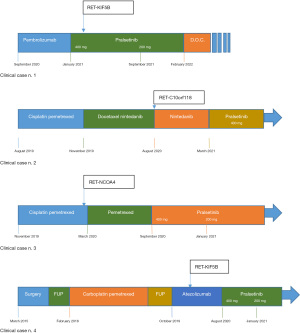
Clinical characteristics are summarized in Table 1. Radiological response is represented in Figure 1, treatment timeline and molecular asset are represented in Figure 2. We present the following cases in accordance with the CARE reporting checklist (available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-50/rc).
Table 1
| Clinical case | Age, years | Sex | Smoking habitus | Previous therapeutic line | RET fusion | Best response to pralsetinib |
|---|---|---|---|---|---|---|
| Case 1 | 78 | Female | Former smoker | 1 | RET-KIF5B | PR |
| Case 2 | 59 | Male | Former smoker | 2 | RET-C10orf118 | PR |
| Case 3 | 63 | Female | Never smoker | 1 | RET-NCOA4 | PR |
| Case 4 | 54 | Female | Never smoker | 2 | RET-KIF5B | PR |
RET, REarranged during Transfection; PR, partial response.
Case presentation
Clinical case 1
A 78-year-old Caucasian woman, former smoker, was diagnosed with metastatic lung adenocarcinoma in July 2020. PD-L1 immunohistochemistry (IHC) expression on tumor sample was 70%. No actionable mutations on EGFR, ALK and ROS1 genes were detected. The patient received first-line treatment with immunotherapy, pembrolizumab 200 mg every 3 weeks. After three months, a full-body computed tomography (CT) scan showed pleural disease progression. Comprehensive molecular profiling using NGS panel (Foundation Medicine, Cambridge, MA, USA) revealed RET-KIF5B fusion. Therefore, in January 2021 the patient started pralsetinib 400 mg/die. Mild hypertension and dysgeusia occurred during treatment but no discontinuation or dose reduction was required. We observed a rapid improvement of respiratory symptoms. In March 2021, CT scan showed a significant partial response in both nodes and pleural disease (Figure 1A). In September 2021 dose reduction (200 mg/die) was needed due recurrent mucositis and fatigue. Last CT scan performed in January 2022 showed stable disease. Patient died in February 2022 for cardiovascular event, not directly related to the disease and pralsetinib treatment.
Clinical case 2
In August 2019, a 59-year-old Caucasian man, former smoker, was diagnosed with lung adenocarcinoma with lung, nodes, adrenal and peritoneum metastases. The patient experienced progressive disease after four cycles of first-line platinum-based doublet chemotherapy. In November 2019, he started a second-line chemotherapy with Nintedanib plus docetaxel, achieving long-lasting control of disease. RET-C10orf118 fusion was detecting thanks NGS panel on tissue biopsy (Foundation Medicine). In March 2021, CT scan showed progressive disease and, then, the patient started treatment with pralsetinib 400 mg/die. CT scan performed after 3 month of treatment showed partial response (Figure 1B). To this date (April 2022), the patient is receiving this treatment reporting clinical benefit and no relevant adverse events.
Clinical case 3
A 63-year-old Caucasian woman, never smoker, was diagnosed with lung adenocarcinoma and multiple bone metastases in August 2019. No druggable mutations were found, PD-L1 IHC expression was 3% on tumor cells. In November 2019, the patient started a chemotherapy treatment with Cisplatin plus Pemetrexed. CT scan, performed after IV cycles of chemotherapy, showed partial response and the patient continued maintenance therapy with pemetrexed until September 2020, when a skeletal scintigraphy showed a progressive disease. NGS analysis (Foundation Medicine), performed with liquid biopsy, revealed a RET-NCOA4 fusion. Therefore, the patient started pralsetinib 400 mg/die. The first radiological re-assessment, performed in December 2020, showed partial response. After 5 months of treatment, the patient experienced olecranon bursitis, mucositis and grade 4 neutropenia [according to Common Terminology Criteria for Adverse Events (CTCAE) v5]. For this reason, patients stopped treatment and resumed it after recovery with reduced pralsetinib dosage to 200 mg/die. In April 2022, patient has had no other significant toxicities and continues treatment with pralsetinib while maintaining clinical benefit and stable radiological disease (Figure 1C).
Clinical case 4
A 54-year-old Caucasian woman, underwent left superior lobectomy for lung adenocarcinoma in march 2015. She received adjuvant chemotherapy with Cisplatin plus Gemcitabine and subsequent radiotherapy. In December 2017, a PET CT scan showed pulmonary relapse, multiple nodes and bone metastases. PD-L1 IHC expression was 10%. Tumor sample resulted wild type for EGFR, ALK and ROS1 mutations. The patient received first-line chemotherapy with Carboplatin plus pemetrexed, and needed bone radiotherapy with palliative intent. After chemotherapy failure, the patient started immunotherapy with atezolizumab. In June 2020, NGS on liquid biopsy (Foundation Medicine) detected RET-KIF5B fusion. In August 2020, CT scan showed ubiquitous progressive disease and the patient started pralsetinib. During treatment, the patient developed grade 3 neutropenia, grade 2 anemia and asthenia, leading to reduce dosage at 200 mg/die. CT scan showed partial response (Figure 1D). Patient in April 2022 is still receiving treatment maintaining clinical benefit and objective response.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Informed consents were obtained from the patients’ relatives/parents. A copy of the written consent is available for review by the editorial office of this journal. Local ethics committee approval was not required due to non-experimental content of the manuscript.
Discussion
In last decades, targeted therapies have dramatically changed the treatment landscape of NSCLC treatment. The advent of novel molecular profiling tools such as NCS led to discovery of new oncogene drivers. Among these, RET fusions, albeit rare, are amenable targets for TKIs. According recent evidence, selpercatinib and pralsetinib have a strong antitumor effect, similar to the more well-known target therapies used against EGFR, ALK and ROS-1 aberrations (3,4,6,15,17). However, NSG is not routinely used in current clinical practice and only a few centers can benefit from it. Similarly, in several countries, pralsetinib and selpercatinib are not yet standard of care in patients with RET-positive NSCLC. Consequently, the impact of these treatments has not been adequately investigated in “real-world” studies.
These clinical series proved the significance of looking for RET fusions in order properly tailor patient’s treatment. Indeed, all patients received pralsetinib achieving significant clinical benefit and radiological response after failure of chemotherapy or immunotherapy. Three patients experienced adverse events leading to dose reduction, but none permanently discontinued treatment. Further and large-scale studies are needed in order to explore and evaluate the clinical outcomes of RET-positive NSCLC treated with TKIs.
Our real word experience is consistent in terms of clinical benefit and progression free survival with results from clinical trials (21).
Conclusions
Despite being only a small case report, this report points out the significance of investigating and targeting RET fusions in NSCLC. Patients receiving pralsetinib had significant clinical benefit and lasting objective responses. Further and large-scale studies are needed in order to explore and evaluate the clinical outcomes of RET-positive NSCLC treated with TKIs.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Precision Cancer Medicine, for the series “Uncommon Mutations in Non-Small Cell Lung Cancer”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-50/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-50/coif). The series “Uncommon Mutations in Non-Small Cell Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. MR served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Precision Cancer Medicine from August 2020 to July 2022. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Informed consents were obtained from the patients’ relatives/parents. A copy of the written consent is available for review by the editorial office of this journal. Local ethics committee approval was not required due to non-experimental content of the manuscript.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yuan M, Huang LL, Chen JH, et al. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal Transduct Target Ther 2019;4:61. [Crossref] [PubMed]
- König D, Savic Prince S, Rothschild SI. Targeted Therapy in Advanced and Metastatic Non-Small Cell Lung Cancer. An Update on Treatment of the Most Important Actionable Oncogenic Driver Alterations. Cancers (Basel) 2021;13:804. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Planchard D, Smit EF, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol 2017;18:1307-16. [Crossref] [PubMed]
- Shaw AT, Riely GJ, Bang YJ, et al. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): updated results, including overall survival, from PROFILE 1001. Ann Oncol 2019;30:1121-26. [Crossref] [PubMed]
- Tsuta K, Kohno T, Yoshida A, et al. RET-rearranged non-small-cell lung carcinoma: a clinicopathological and molecular analysis. Br J Cancer 2014;110:1571-8. [Crossref] [PubMed]
- Ekman S. HER2: defining a Neu target in non-small-cell lung cancer. Ann Oncol 2019;30:353-5. [Crossref] [PubMed]
- Liang H, Wang M. MET Oncogene in Non-Small Cell Lung Cancer: Mechanism of MET Dysregulation and Agents Targeting the HGF/c-Met Axis. Onco Targets Ther 2020;13:2491-510. [Crossref] [PubMed]
- Arbour KC, Rizvi H, Plodkowski AJ, et al. Treatment Outcomes and Clinical Characteristics of Patients with KRAS-G12C-Mutant Non-Small Cell Lung Cancer. Clin Cancer Res 2021;27:2209-15. [Crossref] [PubMed]
- Haratake N, Seto T. NTRK Fusion-positive Non-small-cell Lung Cancer: The Diagnosis and Targeted Therapy. Clin Lung Cancer 2021;22:1-5. [Crossref] [PubMed]
- Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med 2012;18:378-81. [Crossref] [PubMed]
- Kohno T, Ichikawa H, Totoki Y, et al. KIF5B-RET fusions in lung adenocarcinoma. Nat Med 2012;18:375-7. [Crossref] [PubMed]
- Hess LM, Han Y, Zhu YE, et al. Characteristics and outcomes of patients with RET-fusion positive non-small lung cancer in real-world practice in the United States. BMC Cancer 2021;21:28. [Crossref] [PubMed]
- Gainor JF, Curigliano G, Kim DW, et al. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study. Lancet Oncol 2021;22:959-69. [Crossref] [PubMed]
- Login @ Www.Nccn.Org. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Drilon A, Oxnard GR, Tan DSW, et al. Efficacy of Selpercatinib in RET Fusion-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2020;383:813-24. [Crossref] [PubMed]
- A Study Evaluating the Efficacy and Safety of Multiple Therapies in Cohorts of Participants with Locally Advanced, Unresectable, Stage III Non-Small Cell Lung Cancer (NSCLC). [cited 2022 Apr 27]. Available online: https://www.clinicaltrials.gov/ct2/show/NCT05170204?term=PRALSETINIB&draw=2&rank=6
- A Study of Pralsetinib Versus Standard of Care for First-Line Treatment of Advanced Non-Small Cell Lung Cancer (NSCLC). [cited 2022 Apr 27]. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04222972?term=PRALSETINIB&draw=2&rank=4
- Zhou N, Li T, Liang M, et al. Use of Pralsetinib as Neoadjuvant Therapy for Non-Small Cell Lung Cancer Patient With RET Rearrangement. Front Oncol 2022;12:848779. [Crossref] [PubMed]
- Curigliano G, Gainor JF, Griesinger F, et al. Safety and efficacy of pralsetinib in patients with advanced RET fusion-positive non-small cell lung cancer: Update from the ARROW trial. J Clin Oncol 2021;39:9089. [Crossref]
Cite this article as: Galletti A, Russano M, Citarella F, Di Fazio G, Santo V, Brunetti L, Vincenzi B, Tonini G, Santini D. RET-mutated non-small cell lung cancer treated with pralsetinib: a case series. Precis Cancer Med 2022;5:20.

