
KRAS G12V mutation as an acquired resistance mechanism after treatment with dabrafenib and trametinib in non-small cell lung cancer harbouring the BRAF V600E mutation: a case report
Introduction
Recently, molecular-targeted therapies for non-small cell lung cancer (NSCLC) have made remarkable progress. One of the oncogenic drivers in NSCLC is mutated BRAF, a serine-threonine kinase that belongs to the RAF kinase family and that interacts directly with the MEK-ERK signaling cascade. BRAF mutations have been reported in 3–4% of NSCLC patients (1,2), with the BRAF V600 mutation representing about half of all BRAF mutations (3).
Combined treatment with the BRAF inhibitor dabrafenib and the MEK inhibitor trametinib in patients with metastatic BRAF V600E-mutated NSCLC reportedly resulted in an overall response rate (ORR) of 64%, a progression-free survival (PFS) period of 10.9 months and an ORR of 63% in a first-line setting, and a PFS of 9.7 months in subsequent-line settings (4,5); as a result, combined treatment with dabrafenib and trametinib is the current standard of treatment for such patients. Similar to the situation for metastatic EGFR-mutated NSCLC, however, the response duration is limited, and the acquisition of resistance is expected.
Resistance mechanisms to dabrafenib monotherapy or combined dabrafenib and trametinib therapy have been reported in several patients with BRAF V600E-mutated NSCLC; these mechanisms appear to involve MEK1 K57, KRAS G12D, KRAS G12V, KRAS Q61R, NRAS Q61K, and NRAS Q61R mutations (6-9). However, the mechanism of resistance to combined dabrafenib and trametinib therapy in patients with NSCLC has not yet been described adequately.
Here, we describe an acquired resistance mechanism involving KRAS G12V in a patient with BRAF V600E-mutated NSCLC who was treated with a combination of dabrafenib and trametinib. We present the following case in accordance with the CARE reporting checklist (available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-55/rc).
Case presentation
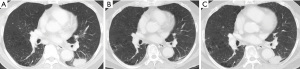
The patient was a 70-year-old Asian male who was a former smoker and has been diagnosed with stage IV NSCLC, adenocarcinoma subtype. Of the left lung since 2018 with metastases to multiple mediastinal lymph nodes and a separate nodule in the counter lateral lung. An endobronchial ultrasound-guided transbronchial needle aspiration biopsy of the left hilar node revealed adenocarcinoma. Initial molecular testing was negative for epidermal growth factor mutations, anaplastic lymphoma kinase (ALK) and ROS1 rearrangements. Subsequent, tissue-based next generation sequencing (NGS) with Oncomine Dx Target Test CDx revealed BRAF V600E mutation, and FoundationOne Liquid by clinical trial setting also showed BRAF V600E and TP53 I255S mutations. He was treated with dabrafenib and trametinib combination therapy and achieved a response, with reduction in size of the left lower lobe primary, mediastinal nodes and multiple lung metastases. In January 2021, he experienced disease progression of the primary lung tumor and multiple new lung nodules in the right lobe (Figure 1). Plasma sequencing (Guardant360® CDx) revealed the acquisition of the KRAS G12V mutation (0.3% of variant allele frequency) in addition to the original BRAF V600E and TP53 I255S mutations (0.2% for both of variant allele frequency). No other genomic mechanism of resistance to dabrafenib and trametinib was identified. Upon disease progression, the patient has been treated with carboplatin and pemetrexed. The response for carboplatin and pemetrexed was partial response with the primary tumour shrinkage.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The number of NSCLC patients harbouring BRAF V600E mutation is relatively small, and only a few cases report on acquired resistance mechanisms have been reported. The identification of recurrent molecular aberrations will lead to the development of new therapeutic strategies for this patient population.
Similar to other advanced NSCLC harbouring actionable mutations treated with targeted agents, resistance almost invariably develop. Multiple mechanisms of resistance to combined BRAF and MEK inhibitors have been reported in BRAF mutant melanoma. These include secondary mutations of the RAS/RAF/MAPK pathway such as BRAF amplification, BRAF splicing variants and gain-of-function mutations in KRAS and NRAS (10). KRAS is upstream from BRAF, and its activation may lead to activation of the PI3K/AKT/mTOR pathway, leading to tumour progression.
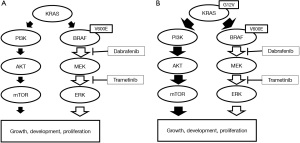
In the presently reported patient, secondary KRAS G12V mutation was identified as mechanism of resistance to combination therapy with dabrafenib and trametinib in our BRAF V600E mutation positive NSCLC patients. Seven cases with acquired resistance to dabrafenib monotherapy or combined dabrafenib and trametinib therapy have been reported (6-9), one of which involved the KRAS G12V mutation. In the literature review, the increased expression level of cyclin-dependent kinase (CDK) 4 and CDKN2 deletion or nonsense mutations have been reported as the resistance mechanisms of non-RAS/RAF/MAPK aberrations (9,11). The present case suggests that the KRAS G12V mutation was present in a minute subclone and that the combination therapy caused the selective outgrowth of the KRAS G12V-positive subclone, leading to tumor progression through the activation of the PI3K/AKT/mTOR pathway (Figure 2). A limitation of our case was that we could not perform molecular analysis by tumor tissue-based assay at resistance. Plasma-based NGS assays cannot rule out the clonal hematopoiesis of indeterminate potential. However, our patient had no KRAS mutation in plasma-based NGS assays before treatment, but plasma-based NGS assays at resistance detected KRAS mutation. For that, we suggest that this KRAS mutation at resistance would be not related to clonal hematopoiesis.
To summarize, we have identified a mechanism of resistance to combined dabrafenib and trametinib therapy involving a KRAS G12V mutation in a patient harboring the BRAF V600E mutation. The optimal approach to overcoming this resistance remains unclear, and further efforts to develop novel treatment strategies and drug combinations are needed to overcome drug resistance.
Acknowledgments
We thank LC-SCRUM-Asia and its operation staff for the gene analysis.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-55/rc
Peer Review File: Available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-55/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-55/coif). KY serves as an unpaid editorial board member of Precision Cancer Medicine from September 2019 to August 2023. KY reports research support from AstraZeneca, Eli Lilly, Pfizer, Daiichi Sankyo, Abbvie, Taiho, Takeda and MSD, and personal fees (honoraria) from AstraZeneca, Bristol-Myers Squibb, Chugai, Daiichi Sankyo, Janssen, Eli Lilly, Taiho, Novartis, Kyowa Kirin and Boehringer Ingelheim. SM reports research support from Chugai, Novartis, Eli Lilly, Merck and MSD, and personal fees (honoraria) from AstraZeneca, Chugai, Novartis, Pfizer and Eli Lilly. HI reports research support from Amgen and personal fees (honoraria) from Ono. YS reports research support from MSD, and personal fees (honoraria) from Ono, Pfizer, Chugai, Novartis, Eli Lilly, Bristol-Myers Squibb, AstraZeneca and Taiho. TS reports personal fees (honoraria) from Chugai and AstraZeneca. KN reports research support from Chugai and Takeda, and personal fees (honoraria) from Pfizer, Chugai, Takeda, Novartis and AstraZeneca. YZ reports research support from AstraZeneca, MSD, Daiichi-Sankyo and personal fees (honoraria) from Pfizer, Chugai, Takeda, AstraZeneca, Bristol-Myers Squibb, Ono pharmaceutical, Boheringer-Ingelheim, Taiho, Novartis, MSD and Eli Lilly. KG reports research support from Boehringer Ingelheim, Bristol-Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Eli Lilly, Guardant Health, Janssen, Kyowa Kirin, Life Technologies Japan, MSD, Novartis, Ono, Otsuka, Pfizer, Taiho and Takeda, and personal fees (honoraria) from Bristol-Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Eli Lilly, Haihe Biopharma, Ignyta, Janssen, KISSEI, Kyowa Kirin, LOXO Oncology, Medical & Biological Laboratories, Merck Biopharma, Merus, MSD, Ono, Pfizer, Sumitomo Dainippon Pharma, Shanghai Haihe, Sysmex Corporation, Taiho, Takeda, and Xcoo. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002;417:949-54. [Crossref] [PubMed]
- Paik PK, Arcila ME, Fara M, et al. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol 2011;29:2046-51. [Crossref] [PubMed]
- Cardarella S, Ogino A, Nishino M, et al. Clinical, pathologic, and biologic features associated with BRAF mutations in non-small cell lung cancer. Clin Cancer Res 2013;19:4532-40. [Crossref] [PubMed]
- Planchard D, Smit EF, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol 2017;18:1307-16. [Crossref] [PubMed]
- Planchard D, Besse B, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol 2016;17:984-93. [Crossref] [PubMed]
- Rudin CM, Hong K, Streit M. Molecular characterization of acquired resistance to the BRAF inhibitor dabrafenib in a patient with BRAF-mutant non-small-cell lung cancer. J Thorac Oncol 2013;8:e41-2. [Crossref] [PubMed]
- Niemantsverdriet M, Schuuring E, Elst AT, et al. KRAS Mutation as a Resistance Mechanism to BRAF/MEK Inhibition in NSCLC. J Thorac Oncol 2018;13:e249-51. [Crossref] [PubMed]
- Abravanel DL, Nishino M, Sholl LM, et al. An Acquired NRAS Q61K Mutation in BRAF V600E-Mutant Lung Adenocarcinoma Resistant to Dabrafenib Plus Trametinib. J Thorac Oncol 2018;13:e131-3. [Crossref] [PubMed]
- Facchinetti F, Lacroix L, Mezquita L, et al. Molecular mechanisms of resistance to BRAF and MEK inhibitors in BRAFV600E non-small cell lung cancer. Eur J Cancer 2020;132:211-23. [Crossref] [PubMed]
- Cohen JV, Sullivan RJ. Developments in the Space of New MAPK Pathway Inhibitors for BRAF-Mutant Melanoma. Clin Cancer Res 2019;25:5735-42. [Crossref] [PubMed]
- Hirai N, Hatanaka Y, Hatanaka KC, et al. Cyclin-dependent kinase 4 upregulation mediates acquired resistance of dabrafenib plus trametinib in BRAF V600E-mutated lung cancer. Transl Lung Cancer Res 2021;10:3737-44. [Crossref] [PubMed]
Cite this article as: Oi H, Yoh K, Matsumoto S, Izumi H, Shibata Y, Sakai T, Nosaki K, Zenke Y, Goto K. KRAS G12V mutation as an acquired resistance mechanism after treatment with dabrafenib and trametinib in non-small cell lung cancer harbouring the BRAF V600E mutation: a case report. Precis Cancer Med 2022;5:18.

