
Current evidence of BRCA mutations in genitourinary and gynecologic tumors: a scoping review
Introduction
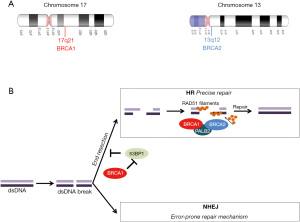
Mutations in the tumor suppressor genes breast cancer genes 1 (BRCA1, 17q21, 113705 OMIM) and 2 (BRCA2, 13q12.3, 600185 OMIM) (Figure 1A) are associated with a significant increased risk of particular types of epithelial malignancies (1). Both genes are inherited in an autosomal dominant fashion, they encode proteins that are part of the homologous recombination (HR) repair pathway (Figure 1B), and that are actively involved in the DNA damage repair (DDR) process (2-4). Therefore, functional BRCA1 and BRCA2 proteins have a crucial role in the repair of double-stranded DNA breaks (5). Hereditary components, such as mutations in BRCA1 and BRCA2, have been found to account for around 5% to 10% of all breast cancers (6). Among hereditary breast cancers, germline mutations in BRCA1 and BRCA2 account for around 30% of all cases; they are mainly associated with early-onset breast cancer, bilateral breast cancer, triple negative (ER, PR and HER2 negative) breast cancer, with the major feature being strong familial history of breast cancer (7,8). Apart from breast and ovarian cancers, mutations in BRCA1 and BRCA2 are associated with an increased risk for cancers of the uterine tubes and peritoneum cancers, while BRCA2 mutations are linked to an increased risk for male breast cancer as well as pancreatic cancer, PC and melanoma (9). All cancers, correlated with a confirmed germline BRCA mutation, are part of the “hereditary breast and ovarian cancer” (HBOC) syndrome (9,10). It has been reported that HBOC patients are also at an increased risk for developing other types of neoplasms such as PC, gastric cancer, pancreatic cancer and melanoma (11).
In this review, we attempt to provide a comprehensive review of the literature on BRCA-mutated ovarian, prostate and endometrial cancers (EC), while highlighting the prevalence and prognostic role of BRCA mutations in genitourinary and gynecologic cancers. We also outline the therapeutic implications and the potent role of PARP inhibitors in the aforementioned genitourinary and gynecologic cancers. The authors present the following article in accordance with the Narrative Review reporting checklist (available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-47/rc).
Methods
Systematic literature search was conducted in PubMed using the terms: BRCA, BRCA1 and BRCA2, ovarian cancer, endometrial cancer, prostate cancer, PARP inhibitors. Only English language articles were included (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search (specified to date, month and year) | August to November 2021 |
| Databases and other sources searched | PubMed |
| Search terms used (including MeSH and free text search terms and filters) | BRCA, BRCA1, BRCA2, ovarian cancer, endometrial cancer, prostate cancer and PARP inhibitors |
| Timeframe | From inception until November 2021 |
| Inclusion and exclusion criteria (study type, language restrictions, etc.) | Inclusion criteria: studies published in English and including the search terms used |
| Selection process (who conducted the selection, whether it was conducted independently, how consensus was obtained, etc.) | AC, ER and SB conducted the data selection |
Ovarian cancer and BRCA
Ovarian cancer (OC) is the eighth most common cancer in women worldwide, and to date the majority (75–80%) of patients are diagnosed at an advanced stage of the disease (12,13). It represents the second most common gynecological malignancy with a worldwide incidence of around 225,000 women per year (14).
Over the last two decades, the standard of care for women with advanced OC has been a combination of cytoreductive surgery and systemic platinum-based chemotherapy. However, most of the patients with advanced disease have unfortunately, a high risk of relapse within 3 years into the treatment (15). Most OC are part of the autosomal dominantly-inherited cancer-predisposition syndrome HBOC that predisposes to breast and ovarian cancers. HBOC syndrome stems from germline mutations mainly in BRCA1 or BRCA2 (11). Heterozygous carriers of BRCA1 or BRCA2 mutations have an increased lifetime risk of developing OC, with an estimated likelihood of 40–60% for BRCA1 and 11–30% for BRCA2 (16,17). Germline mutations in BRCA genes have been reported in several histological subtypes of OC (Endometrioid, clear cell) with the highest rates of mutations reported in high-grade serous OC (18-21). The prevalence of BRCA1/2 mutations in OC vary between 10 and 25% depending on the OC subtype (22-25). The presence of a germline BRCA mutation in high-grade serous OC patients confers a survival benefit when compared to patients with no germline mutation (26). BRCA2 mutation carriers, in particular, have a higher survival rate probably due to the role of BRCA2 protein in regulating the process by which the crosslink damage repair occurs (27).
The histologic, molecular, and genetic evidences unveiled over the last decade show that the majority of the fallopian tube cancers and primary peritoneal carcinoma should be considered collectively a single entity and treated similarly to OC (28). In 2014, the FIGO’s Committee for Gynecologic Oncology revision of the staging of ovarian cancer incorporated ovarian, fallopian tube, and peritoneal cancer into the same system (29). These three entities were traditionally included in the same pivotal trials of PARP inhibitors however, a recent SEER analysis showed a differential effect of the treatment eras across the different tumors (30). The findings of this study cannot be over-interpreted because of its retrospective methodology and the inherent limitations to registry analysis. Subgroup analyses of the phase III randomized controlled trials, by tumor subset, would shed more light on the differential effects of PARP inhibitors.
It is currently well established that BRCA-associated OC display distinct clinical characteristics with a relatively earlier age at diagnosis, improved survival, visceral disease, and higher response rates to specific chemotherapies and other types of treatments (discussed in the section “Targeting BRCA-mutated cancers” of this review) (13,31).
EC and BRCA
EC is the fifth most common female cancer in developed countries (32). EC can be divided into two histological categories, with different incidence and prognosis: type I (80% of cases) including low grade tumors with a relatively good prognosis and type II (20% of cases) including high grade endometroid tumors with a relatively poor prognosis (32,33). Numerous risk factors are reported to be linked to EC including non-genetic (i.e., exposure to estrogens, menarche at early age, late onset menopause, obesity etc.); and genetic factors reported in several familial cases (34,35).
Hereditary EC is part of 3 different syndromes: Lynch syndrome, Cowden syndrome and HBOC syndrome (35-37). Lynch syndrome is caused by dominant mutations in DNA mismatch repair (MMR) genes such as MutL Homolog 1 (MLH1), MutS Homolog 2 (MSH2), MutS Homolog 6 (MSH6) and PMS1 Homolog 2 (PMS2); patients who carry a germline mutation in one of these MMR genes have a 20–70% cumulative lifetime risk to develop EC, particularly women with mutations in MSH2 or MLH1 (36-38). Cowden syndrome is a rare condition stemming from a mutation in the tumor suppressor gene Phosphatase and Tensin Homolog (PTEN), it is characterized by the development of tumors in multiple organs and includes an increased risk for EC (35). While HBOC patients have an increased likelihood to develop EC, the classification of EC as part of HBOC is still debatable, although, new evidence favoring EC as part of the BRCA-associated HBOC syndrome with unfavorable clinical outcome, has been reported (39), which might have important implications on the treatment strategies and clinical management of EC patients.
In most data reported on EC with germline BRCA mutations, the incidence of developing EC was assessed by age group, ethnicity or EC subtypes showing a slightly increased risk of EC in mutated-BRCA carriers, mainly BRCA1. In 2019, a multinational cohort study involving more than 11,000 BRCA1 mutation carriers, clearly outlined the association between BRCA1 mutations and the risk for EC (40). This was the earliest report linking BRCA mutations to the risk of developing EC. Further studies also reported high rates of BRCA1 and BRCA2 mutation carriers, in Jewish women with papillary serous uterine cancer (41-43). Another study, reported that the main contributor to the increased risk of EC among BRCA mutation carriers is tamoxifen exposure (44). The data was further corroborated to a lesser extent in another prospective study (45). On the other hand, studies disputing any association between BRCA mutations and the risk of developing EC were reported (46,47), namely a large cohort study of 1,170 cases of EC showing low incidence of germline BRCA1/2 mutations in EC type I, type II and uterine serous cancers (48). A recent meta-analysis evaluating the risk of EC in BRCA1 or BRCA2 germline mutation carriers, reported that the prevalence rate of EC in BRCA1/2 mutations carriers was 0.59% (49). In these studies, EC prevalence was 0.62% among BRCA1 mutation carriers and 0.47% among BRCA2 mutation carriers (49).
PC and BRCA
PC is the second most common neoplasm in men worldwide (50). In spite of all the advances achieved in PC care, the clinical outcome of patients with metastatic castration-resistant prostate cancers (mCRPC) is poor and the median overall survival (OS) still unsatisfactory (51). PC is ranked among the most heritable human cancers, a large proportion of mCRPC patients carry potentially actionable germline and somatic genetic variants, with BRCA2 mutations representing the most common alteration (52-55).
Molecular studies have shown that genomic landscapes and patterns are different between mCRPC and localized PC (53,56,57). For instance, the incidence of germline mutations in DDR genes among men with metastatic PC reportedly ranges between ~11% and ~33%, which is significantly higher than in men with localized PC (56,57). Among the DDR defected genes, BRCA2 is the most frequently mutated, followed by genes with lower mutation frequency such as ATM, TP53, CDL12, CHEK2, BRCA1, FANCA, RAD51, MLH1 and other genes (56-58). A large study investigating 692 patients with metastatic PC, the prevalence of BRCA2 mutations was 5.3% and BRCA1 mutations was 0.9% (57). Several retrospective studies suggested a strong association between BRCA2 mutations and PC risk with a 2 to 6 folds elevated risk compared to men in the general population; while BRCA1 mutations are mianly associated with a moderate risk of PC at younger ages (59-64). BRCA2 mutations are considered as strong independent negative prognostic factors in patients with mCRPC, and are associated with short metastasis-free survival and cancer-specific survival (65,66). Furthermore, BRCA mutations are frequently diagnosed at an advanced stage (T3/T4), associated with nodal involvement and metastatic disease (65).
Conflicting results reported in several retrospective studies, made it unclear whether BRCA2 mutations could affect the outcome of mCRPC patients treated according to standard recommendations (67-69). Reports have suggested that the choice of first-line therapy is the main factor that may affect the outcome for germline mutated BRCA2 patients (70). PC is a clinically-heterogeneous disease, with patients responding variably to treatments leading to different outcomes; probably because of the molecular heterogeneity of PC cells. Therefore, molecular profiling could be of a great benefit, allowing the detection of BRCA mutations that can predict response to treatments such as the Poly-ADP ribose polymerase (PARP) inhibitors and the platinum agents. The therapeutic implication of BRCA mutations in PC will be detailed in the following section.
Targeting BRCA-mutated cancers
BRCA1/2 mutations are biomarkers that have an important role in the selection of treatment for breast and other cancers. Tumors that arise in individuals with a BRCA mutation have a homologous repair deficiency (HRD) (Figure 1B) (5), which may cause the cells to be sensitive to platinum-based chemotherapies and PARP inhibitors. In 2005, it was reported by two independent research groups that BRCA-deficient cancers are sensitive to PARP inhibition, uncovering the synthetic lethal interaction process that takes place between PARP inhibition and BRCA mutations (71,72).
Platinum-based chemotherapy
Platinum-based chemotherapy activity relies on its ability to interfere with DNA repair mechanisms leading to DNA damage and apoptosis in different cancer types. Whenever the tumor cells DNA repair mechanism is altered, the responses to platinum chemotherapy are enhanced (73). These cytotoxic drugs kill cancer cells through DNA damaging, DNA synthesis and mitosis inhibition; they also induce apoptosis (73).
Platinum-based anticancer agents have been extensively explored in numerous clinical trials, and have currently a wide spectrum of clinical application either as monotherapy or in combination with other chemotherapeutic agents, mainly in mCRPC but also in hormone-sensitive diseases (74,75). OC with a germline mutant BRCA1 or BRCA2 has a greater sensitivity to platinum-based treatment, as well as an improved OS compared to non-BRCA-related OC. However, despite initial high response rates to platinum and taxane-based first line chemotherapy, most OC patients would relapse, with a median progression-free survival (PFS) of 18 months (76).
Furthermore, relapses are followed by a substantial decrease in sensitivity to platinum-based chemotherapy resulting in the development of a resistance to platinum agents and the subsequent platinum-refractory disease, which is characterized by a progression of the disease within 6 months of platinum treatment initiation, and which usually has a very poor prognosis (77).
PARP inhibitors
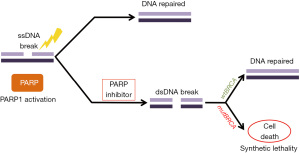
PARP inhibitors rely on the concept of synthetic lethality in BRCA-deficient tumors through their inability to repair double-stranded DNA breaks, which leads to cell death (Figure 2) (78). In normal cells, PARP family of enzymes repair DNA damage at the site of single strand breaks through HR, base excision repair (BER) or nucleotide excision repair (NER) mechanisms (79). The concept of synthetic lethality describes a situation where a mutation in either of two genes individually has no serious effect, but where the combination of mutations leads to cell death; this was demonstrated in cell lines with BRCA1 or BRCA2 mutations (71,72). The mechanism behind the synthetic lethal interaction between PARP inhibition and loss of BRCA function is thought to be related to the accumulation of single-stranded breaks, that would block the replication fork and lead to double strand breaks (5,78). Normal cells have the potential to repair double strand breaks and stay viable however, cancer cells with HRD are unable to do so. In the context of cancer cells with non-functional BRCA, it is thought that double strand breaks accumulate leading to highly toxic genomic lesions and instability, and finally cell death (5,78,80).
Therapeutic inhibitors of this pathway were first explored and the results published in 2009, in a phase I study (NCT00516373) on olaparib. The study demonstrated the anti-tumor activity of olaparib in cancers harboring BRCA mutations, with fewer adverse effects compared to conventional chemotherapy (31). In 2014, the European medicines agency (EMA) granted approval to olaparib in a maintenance setting for patients carrying BRCA mutations with recurrent high-grade serous epithelial OC, fallopian tube or primary peritoneal carcinoma (81). Few months later, the US food and drug administration (FDA), approved olaparib for the same aforementioned cancers, in patients with germline BRCA mutations previously treated with three lines or more of chemotherapy (82). Recently, a 5-year follow up phase III (NCT01844986) trial reported that the benefit derived from 2 years maintenance therapy with olaparib was sustained beyond the end of treatment, with an extended median PFS (56 months) (83).
Following olaparib’s approval (NCT01874353), a remarkable improvement in PFS was reported in two randomized phase III clinical trials between 2016 and 2017, which led to the approval of rucaparib and niraparib (NCT01847274 and NCT01968213) as maintenance therapy for complete or partial platinum-sensitive recurrent BRCA-mutated ovarian cancers (84-86). The FDA and EMA approved the use of rucaparib in 2016 and 2018, respectively, for the treatment of high-grade serous epithelial OC, fallopian tube or primary peritoneal carcinoma with germline or somatic BRCA mutations, after two or more lines of chemotherapies (87-89). In 2017, the FDA and EMA approved niraparib as a maintenance treatment in patients achieving complete or partial remission after platinum-based therapy in patients with recurrent epithelial OC, fallopian tube or primary peritoneal carcinoma. In newly diagnosed patients with advanced OC who had a favorable response to platinum-based chemotherapy, niraparib treatment showed a longer PFS, regardless of the presence or absence of HRD (90). It was therefore suggested that resistance to platinum-therapy decreases sensitivity to PARP inhibition; however, another study, showed significant antitumor effect in patients classified with platinum-resistant disease (31,91).
Further testing and data analyses were conducted, upon which, PARP inhibitors were approved not only as maintenance but also as induction therapy, for pretreated recurrent ovarian cancer (82,88,92). Clinical trials further demonstrated notable clinical activity of PARP inhibitors in OC even in the absence of germline BRCA mutations (81,93). This was first reported in a phase II clinical study (NCT00679783) including patients with OC and unknown BRCA status, BRCA-negative or BRCA-positive, treated with olaparib (93). Subsequently, both FDA and EMA approved the use of olaparib as a maintenance therapy of platinum-sensitive recurrent OC regardless of BRCA mutational status (81,86).
Currently, clinical trials on olaparib, nipraparib, rucaparib and talazoparib are being conducted in different settings and disease stage as monotherapy or in novel and standard of care combinations. For example, olaparib as maintenance monotherapy in platinum-sensitive relapsed germline-unmutated-BRCA OC (NCT03402841); niraparib as maintenance therapy in newly-diagnosed OC patients (NCT04986371); rucaparib as maintenance therapy after bevacizumab maintenance following carboplatin-based first-line chemotherapy in OC patients (NCT04227522); talazoparib and radiation therapy in treating patients with locally recurrent gynecologic cancers (NCT03968406), and many other clinical trials.
In PC, several PARP inhibitors are still under investigation especially in mCRPC patients. Research conducted in human PC cell line (DU145), confirmed what is known about olaparib this time in PC cells, confirming the trapping of PARP1 and PARP2 and providing a rationale for further researches (94). Olaparib was the first PARP inhibitor exhibiting significant activity in mCRPC patients. In a phase II clinical trial, among patients with DDR, 88% showed favorable response to olaparib and an improved radiologic PFS and OS (56). Olaparib, nipraparib, rucaparib as well as talazoparib are currently being tested in many ongoing clinical trials (phase I, II or III) in order to assess the role and efficacy of PARP inhibitors in mCRPC (70) (i.e., NCT03874884, NCT02861573, NCT03787680, NCT03732820, NCT03834519, NCT03431350, NCT03748641, NCT03840200, NCT02975934, NCT04019327, NCT03395197, and other trials).
In EC, it is true that preclinical evidence reported sensitivity of PTEN-deficient cells to PARPi; however, to date there is no clinical evidence of activity in PTEN-altered tumors. Data from in vitro studies showed that PTEN-deficiency provides a significantly greater sensitivity to PARP inhibitors (KU0058948 or olaparib) (95,96). Indeed, PTEN-deficient EC cells were reported to be more sensitive to olaparib and talazoparib than wild-type PTEN cell lines in vitro. Furthermore, since the PI3K/mTOR pathway is overactivated in PTEN-mutated cells, the use of PI3K inhibitors reportedly enhanced the sensitivity of these cells to PARP inhibitors (97). Moreover, in vivo studies conducted in mice, showed that PARP inhibitors in combination with hormonal therapy may increase the antitumor efficacy in PTEN-deficient EC (98). In two distinct case reports describing the use of olaparib with EC, the first patient had recurrent EC (PTEN-deficient, somatic BRCA1/2 negative) with brain metastasis, and responded well to olaparib; she, however had progression of the disease 8 months upon the initiation of the treatment (99). The second case had a low-grade EC that relapsed (germline BRCA2-mutated), she received olaparib as a maintenance therapy, and showed a durable response and a stable disease documented for over 15 months (100). Currently, the role and efficacy of olaparib, nipraparib, rucaparib and talazoparib in recurrent, advanced and metastatic EC, as monotherapies or in combination with other therapies (i.e., NCT03745950, NCT03951415, NCT02755844, NCT04065269, NCT03617679, NCT03694262, NCT03552471, NCT04080284, NCT03016338, NCT02127151, NCT03968406, and others) are under evaluation in numerous phase I and II clinical trials.
Additionally, several clinical trials demonstrated significant benefit and improvement of PARP inhibitors, when used in other cancers such as HER2-negative BRCA-mutated breast cancers, and pancreatic cancers (82,101,102). Indeed, in 2018 olaparib and talazoparib were FDA-approved for patients with HER2-negative BRCA-mutated metastatic breast cancer with disease recurrence following chemotherapy (101,102). To date, there is insufficient data on the use of talazoparib for the treatment of OC. Few phase I clinical trials have been carried out on this matter (NCT01286987) (103,104), while other studies are still underway (NCT02316834, NCT02326844). Further studies and clinical trials are still needed, in order to compare the effect of talazoparib in OC with approved PARP inhibitors and to evaluate the benefits of this drug in OC.
Combining PARP inhibitors with other therapies
Combining PARP inhibitors with other therapies was explored in several studies that reported potential augmentation of DNA damages, enabling further antitumor responses. Several therapeutic agents have been studied in combination with PARP inhibitors, including inhibitors of vascular endothelial growth factor (VEGF), and PD-1/PD-L1, anti-CTLA4 monoclonal antibodies, mTOR inhibitor, AKT inhibitor, and PI3K inhibitor, as well as MEK 1/2, and WEE1 inhibitors (105). Current ongoing clinical trials are evaluating the combining effect of PARP inhibitors to other therapeutic agents. For instance, combined PARP inhibitors and immune checkpoint strategy was reported to induce important and efficient toxicity levels. On the other hand, combining olaparib with durvalumab (anti PD-L1) or niraparib with pembrolizumab (anti PD-1) demonstrated promising anti-tumour activity and safety similar to monotherapy strategy (106-108). Furthermore, combining PARP inhibitors with antiangiogenic agents such as olaparib and cediranib, a potent inhibitor of VEGF, showed promising results and a significant longer PFS compared to olaparib alone (109).
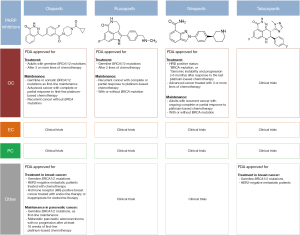
To sum up, among the four FDA-approved PARP inhibitors as monotherapy (Figure 3), to date, only three have been approved for the treatment or maintenance of OC: olaparib, rucaparib and niraparib. Several clinical trials evaluating the use of the FDA-approved PARP inhibitors as well as new molecules, as monotherapy or in combination, for the treatment of genitourinary and gynecologic tumors, are ongoing.
BRCAness, beyond BRCA1 and BRCA2 mutations
Over the last decade, it became clear that a proportion of sporadic cancers, especially HBOC (i.e., ovarian cancer), do not harbor BRCA mutations; although, they share similar pathological and clinical features as BRCA-mutated cancers. This concept was named “BRCAness” and it reflects the presence of a common phenotype between sporadic cancers and familial cancers harboring BRCA mutations (110). BRCAness describes the situation where a homologous recombination DNA repair defect is present with no germline BRCA mutation detected (111). The defective HR observed may be caused by several mechanisms, such as: hypermethylation of BRCA1 promoter, somatic BRCA mutations, or defects in individual genes that can modulate HR repair (ATM, ATR, CHEK1, CHEK2, DSS1, RAD51, NBS1, FANC family of genes) (112). Also, EMSY amplification and PTEN mutation/deletion were reportedly associated with HRD (112); however, other studies reported that the link between EMSY or PTEN genes and HRD phenotype is not established (113,114).
Nowadays, the activity of PARP inhibitors is beyond the presence of germline BRCA mutations and more commonly applicable to HRD OC (90,115). This concept is therapeutically and clinically important. Indeed, in several clinical trials on OC, it was reported that PARP inhibitors show activity despite the absence of BRCA mutations (81,93).
The ability to determine BRCAness has been improved by the recent advances accomplished in the field of molecular profiling of tumors. However, there is still limited activity detected beyond BRCA mutations. Identification of further functional biomarkers of HR repair (HRR) and responses to PARPi (56,116), would potentially expand our knowledge of cancer cells, and validate the utility of innovative targeted therapies such as PARP inhibitors.
Conclusions and perspectives
The booming of research investigating the interactions between cancer genes and potential therapeutic targets, reflects the success behind the discovery and development of the synthetic lethal therapies with PARP inhibitors for patients with BRCA-mutated cancer. To date, PARP inhibitors remain the only FDA-approved therapy using the synthetic lethality approach. Nevertheless, large-scale studies already uncovered additional synthetic lethal interactions that might be of use in targeting cancer cells (117), while other studies are underway for potential discovery of new therapeutic targets along the same concept. The advent of new scientific technologies, such as CRISPR interference (CRISPRi) method or its variant technique “Perturb-Seq” that allows the identification of gene signatures, new components to pathways and gene targets, may be a valuable additional tool that can be used to delineate large-scale genetic interactions (118,119). Altogether, the advances in the molecular and genomic fields as well as the ongoing preclinical and clinical studies will undoubtedly pave the way for the discovery and implementation of new robust approaches in the era of precision medicine.
Identification of a BRCA mutation may not only help the afflicted patient, but it would as well allow genetic counseling and testing to be performed in relatives. Available data from preclinical, clinical and translational studies, enabled ongoing research for the evaluation of different therapeutic strategies such as combinations of PARP inhibitors and immune checkpoint inhibitors: PD-1/PD-L1 inhibitors in OC (NCT04191135, NCT03911453), and PI3K inhibitors in OC, PC and EC (NCT04586335); or association of PARP inhibitors and anti-angiogenic agents (i.e., NCT04566952). Outcoming results from several ongoing clinical trials would provide more comprehensive data on the clinical benefit of new drugs and new combinations (120).
An important non negligible factor however, is the resistance to PARP inhibitors. Previous studies reported that resistance may occur through different cellular mechanisms such as the restoration of homology-directed DNA repair as a result of BRCA reversions, or through the loss of 53BP1 (an important preclinical finding) by mutation/downregulation (121,122). Detection of these biomarkers and further identification of new ones would enable patient selection for PARP inhibitors. Another approach would be to overcome the acquired resistance to PARP inhibitors, for example by inhibiting CDK12, WEE1 or ATR (123-125). Along with the success accomplished with the development of PARP inhibitors, further studies exploring alternative approaches to overcome drug resistance are still needed in the purpose of widening the scope of clinical trials and shedding light on potential new therapeutical targets.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Precision Cancer Medicine for the series “Precision Oncology in Urogenital and Gynecological Tumors”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-47/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-47/coif). The series “Precision Oncology in Urogenital and Gynecological Tumors” was commissioned by the editorial office without any funding or sponsorship. ER and SB served as the unpaid Guest Editors of the series. ER serves as an unpaid editorial board member of Precision Cancer Medicine from July 2021 to June 2023. MM is currently an employee of Novartis, Basel, Switzerland. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rosen EM, Pishvaian MJ. Targeting the BRCA1/2 tumor suppressors. Curr Drug Targets 2014;15:17-31. [Crossref] [PubMed]
- Lindor NM, McMaster ML, Lindor CJ, et al. Concise handbook of familial cancer susceptibility syndromes - second edition. J Natl Cancer Inst Monogr 2008;(38):1-93.
- Boulton SJ. Cellular functions of the BRCA tumour-suppressor proteins. Biochem Soc Trans 2006;34:633-45. [Crossref] [PubMed]
- Roy R, Chun J, Powell SN. BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nat Rev Cancer 2011;12:68-78. [Crossref] [PubMed]
- Lord CJ, Ashworth A. PARP inhibitors: Synthetic lethality in the clinic. Science 2017;355:1152-8. [Crossref] [PubMed]
- Valencia OM, Samuel SE, Viscusi RK, et al. The Role of Genetic Testing in Patients With Breast Cancer: A Review. JAMA Surg 2017;152:589-94. [Crossref] [PubMed]
- Economopoulou P, Dimitriadis G, Psyrri A. Beyond BRCA: new hereditary breast cancer susceptibility genes. Cancer Treat Rev 2015;41:1-8. [Crossref] [PubMed]
- Sharma P, Klemp JR, Kimler BF, et al. Germline BRCA mutation evaluation in a prospective triple-negative breast cancer registry: implications for hereditary breast and/or ovarian cancer syndrome testing. Breast Cancer Res Treat 2014;145:707-14. [Crossref] [PubMed]
- Grignol VP, Agnese DM. Breast Cancer Genetics for the Surgeon: An Update on Causes and Testing Options. J Am Coll Surg 2016;222:906-14. [Crossref] [PubMed]
- Petrucelli N, Daly MB, Pal T, et al. BRCA1- and BRCA2-Associated Hereditary Breast and Ovarian Cancer 1993.
- Kobayashi H, Ohno S, Sasaki Y, et al. Hereditary breast and ovarian cancer susceptibility genes Oncol Rep 2013;30:1019-29. (review). [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Banerjee S, Kaye SB. New strategies in the treatment of ovarian cancer: current clinical perspectives and future potential. Clin Cancer Res 2013;19:961-8. [Crossref] [PubMed]
- Banerjee S, Kaye SB, Ashworth A. Making the best of PARP inhibitors in ovarian cancer. Nat Rev Clin Oncol 2010;7:508-19. [Crossref] [PubMed]
- Ledermann JA, Raja FA, Fotopoulou C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24:vi24-32. [Crossref] [PubMed]
- Easton DF, Ford D, Bishop DT. Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Am J Hum Genet 1995;56:265-71. [PubMed]
- Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet 1998;62:676-89. [Crossref] [PubMed]
- Risch HA, McLaughlin JR, Cole DE, et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet 2001;68:700-10. [Crossref] [PubMed]
- Pal T, Permuth-Wey J, Betts JA, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer 2005;104:2807-16. [Crossref] [PubMed]
- Zhang S, Royer R, Li S, et al. Frequencies of BRCA1 and BRCA2 mutations among 1,342 unselected patients with invasive ovarian cancer. Gynecol Oncol 2011;121:353-7. [Crossref] [PubMed]
- Soegaard M, Kjaer SK, Cox M, et al. BRCA1 and BRCA2 mutation prevalence and clinical characteristics of a population-based series of ovarian cancer cases from Denmark. Clin Cancer Res 2008;14:3761-7. [Crossref] [PubMed]
- Ledermann JA, Drew Y, Kristeleit RS. Homologous recombination deficiency and ovarian cancer. Eur J Cancer 2016;60:49-58. [Crossref] [PubMed]
- Manchana T, Phoolcharoen N, Tantbirojn P. BRCA mutation in high grade epithelial ovarian cancers. Gynecol Oncol Rep 2019;29:102-5. [Crossref] [PubMed]
- Alsop K, Fereday S, Meldrum C, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol 2012;30:2654-63. [Crossref] [PubMed]
- Abdulrashid K, AlHussaini N, Ahmed W, et al. Prevalence of BRCA mutations among hereditary breast and/or ovarian cancer patients in Arab countries: systematic review and meta-analysis. BMC Cancer 2019;19:256. [Crossref] [PubMed]
- Rubin SC, Benjamin I, Behbakht K, et al. Clinical and pathological features of ovarian cancer in women with germ-line mutations of BRCA1. N Engl J Med 1996;335:1413-6. [Crossref] [PubMed]
- Cipak L, Watanabe N, Bessho T. The role of BRCA2 in replication-coupled DNA interstrand cross-link repair in vitro. Nat Struct Mol Biol 2006;13:729-33. [Crossref] [PubMed]
- Berek JS, Renz M, Kehoe S, et al. Cancer of the ovary, fallopian tube, and peritoneum: 2021 update. Int J Gynaecol Obstet 2021;155:61-85. [Crossref] [PubMed]
- Prat JFIGO Committee on Gynecologic Oncology. FIGO's staging classification for cancer of the ovary, fallopian tube, and peritoneum: abridged republication. J Gynecol Oncol 2015;26:87-9. [Crossref] [PubMed]
- Pavlidis N, Rassy E, Vermorken JB, et al. The outcome of patients with serous papillary peritoneal cancer, fallopian tube cancer, and epithelial ovarian cancer by treatment eras: 27 years data from the SEER registry. Cancer Epidemiol 2021;75:102045. [Crossref] [PubMed]
- Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 2009;361:123-34. [Crossref] [PubMed]
- Johnatty SE, Tan YY, Buchanan DD, et al. Family history of cancer predicts endometrial cancer risk independently of Lynch Syndrome: Implications for genetic counselling. Gynecol Oncol 2017;147:381-7. [Crossref] [PubMed]
- Felix AS, Weissfeld JL, Stone RA, et al. Factors associated with Type I and Type II endometrial cancer. Cancer Causes Control 2010;21:1851-6. [Crossref] [PubMed]
- Win AK, Reece JC, Ryan S. Family history and risk of endometrial cancer: a systematic review and meta-analysis. Obstet Gynecol 2015;125:89-98. [Crossref] [PubMed]
- Shai A, Segev Y, Narod SA. Genetics of endometrial cancer. Fam Cancer 2014;13:499-505. [Crossref] [PubMed]
- Ring KL, Bruegl AS, Allen BA, et al. Germline multi-gene hereditary cancer panel testing in an unselected endometrial cancer cohort. Mod Pathol 2016;29:1381-9. [Crossref] [PubMed]
- Cohen SA, Leininger A. The genetic basis of Lynch syndrome and its implications for clinical practice and risk management. Appl Clin Genet 2014;7:147-58. [Crossref] [PubMed]
- Stoffel E, Mukherjee B, Raymond VM, et al. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. Gastroenterology 2009;137:1621-7. [Crossref] [PubMed]
- de Jonge MM, Ritterhouse LL, de Kroon CD, et al. Germline BRCA-Associated Endometrial Carcinoma Is a Distinct Clinicopathologic Entity. Clin Cancer Res 2019;25:7517-26. [Crossref] [PubMed]
- Thompson D, Easton DFBreast Cancer Linkage Consortium. Cancer Incidence in BRCA1 mutation carriers. J Natl Cancer Inst 2002;94:1358-65. [Crossref] [PubMed]
- Lavie O, Ben-Arie A, Segev Y, et al. BRCA germline mutations in women with uterine serous carcinoma--still a debate. Int J Gynecol Cancer 2010;20:1531-4. [PubMed]
- Biron-Shental T, Drucker L, Altaras M, et al. High incidence of BRCA1-2 germline mutations, previous breast cancer and familial cancer history in Jewish patients with uterine serous papillary carcinoma. Eur J Surg Oncol 2006;32:1097-100. [Crossref] [PubMed]
- Bruchim I, Amichay K, Kidron D, et al. BRCA1/2 germline mutations in Jewish patients with uterine serous carcinoma. Int J Gynecol Cancer 2010;20:1148-53. [Crossref] [PubMed]
- Beiner ME, Finch A, Rosen B, et al. The risk of endometrial cancer in women with BRCA1 and BRCA2 mutations. A prospective study. Gynecol Oncol 2007;104:7-10. [Crossref] [PubMed]
- Segev Y, Iqbal J, Lubinski J, et al. The incidence of endometrial cancer in women with BRCA1 and BRCA2 mutations: an international prospective cohort study. Gynecol Oncol 2013;130:127-31. [Crossref] [PubMed]
- Shu CA, Pike MC, Jotwani AR, et al. Uterine Cancer After Risk-Reducing Salpingo-oophorectomy Without Hysterectomy in Women With BRCA Mutations. JAMA Oncol 2016;2:1434-40. [Crossref] [PubMed]
- Lee YC, Milne RL, Lheureux S, et al. Risk of uterine cancer for BRCA1 and BRCA2 mutation carriers. Eur J Cancer 2017;84:114-20. [Crossref] [PubMed]
- Long B, Lilyquist J, Weaver A, et al. Cancer susceptibility gene mutations in type I and II endometrial cancer. Gynecol Oncol 2019;152:20-5. [Crossref] [PubMed]
- Matanes E, Volodarsky-Perel A, Eisenberg N, et al. Endometrial Cancer in Germline BRCA Mutation Carriers: A Systematic Review and Meta-analysis. J Minim Invasive Gynecol 2021;28:947-56. [Crossref] [PubMed]
- Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2017;3:524-48. [Crossref] [PubMed]
- Gillessen S, Omlin A, Attard G, et al. Management of patients with advanced prostate cancer: recommendations of the St Gallen Advanced Prostate Cancer Consensus Conference (APCCC) 2015. Ann Oncol 2015;26:1589-604. [Crossref] [PubMed]
- Mucci LA, Hjelmborg JB, Harris JR, et al. Familial Risk and Heritability of Cancer Among Twins in Nordic Countries. JAMA 2016;315:68-76. [Crossref] [PubMed]
- Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015;161:1215-28. [Crossref] [PubMed]
- Kote-Jarai Z, Leongamornlert D, Saunders E, et al. BRCA2 is a moderate penetrance gene contributing to young-onset prostate cancer: implications for genetic testing in prostate cancer patients. Br J Cancer 2011;105:1230-4. [Crossref] [PubMed]
- Eeles R, Goh C, Castro E, et al. The genetic epidemiology of prostate cancer and its clinical implications. Nat Rev Urol 2014;11:18-31. [Crossref] [PubMed]
- Mateo J, Carreira S, Sandhu S, et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N Engl J Med 2015;373:1697-708. [Crossref] [PubMed]
- Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N Engl J Med 2016;375:443-53. [Crossref] [PubMed]
- Mateo J, Seed G, Bertan C, et al. Genomics of lethal prostate cancer at diagnosis and castration resistance. J Clin Invest 2020;130:1743-51. [Crossref] [PubMed]
- Oh M, Alkhushaym N, Fallatah S, et al. The association of BRCA1 and BRCA2 mutations with prostate cancer risk, frequency, and mortality: A meta-analysis. Prostate 2019;79:880-95. [Crossref] [PubMed]
- Kirchhoff T, Kauff ND, Mitra N, et al. BRCA mutations and risk of prostate cancer in Ashkenazi Jews. Clin Cancer Res 2004;10:2918-21. [Crossref] [PubMed]
- Agalliu I, Gern R, Leanza S, et al. Associations of high-grade prostate cancer with BRCA1 and BRCA2 founder mutations. Clin Cancer Res 2009;15:1112-20. [Crossref] [PubMed]
- Mersch J, Jackson MA, Park M, et al. Cancers associated with BRCA1 and BRCA2 mutations other than breast and ovarian. Cancer 2015;121:269-75. Erratum in: Cancer 2015;121:2474-5. [Crossref] [PubMed]
- Nyberg T, Frost D, Barrowdale D, et al. Prostate Cancer Risks for Male BRCA1 and BRCA2 Mutation Carriers: A Prospective Cohort Study. Eur Urol 2020;77:24-35. [Crossref] [PubMed]
- Leongamornlert D, Mahmud N, Tymrakiewicz M, et al. Germline BRCA1 mutations increase prostate cancer risk. Br J Cancer 2012;106:1697-701. [Crossref] [PubMed]
- Castro E, Goh C, Olmos D, et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol 2013;31:1748-57. [Crossref] [PubMed]
- Castro E, Romero-Laorden N, Del Pozo A, et al. PROREPAIR-B: A Prospective Cohort Study of the Impact of Germline DNA Repair Mutations on the Outcomes of Patients With Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol 2019;37:490-503. [Crossref] [PubMed]
- Annala M, Struss WJ, Warner EW, et al. Treatment Outcomes and Tumor Loss of Heterozygosity in Germline DNA Repair-deficient Prostate Cancer. Eur Urol 2017;72:34-42. [Crossref] [PubMed]
- Mateo J, Cheng HH, Beltran H, et al. Clinical Outcome of Prostate Cancer Patients with Germline DNA Repair Mutations: Retrospective Analysis from an International Study. Eur Urol 2018;73:687-93. [Crossref] [PubMed]
- Antonarakis ES, Lu C, Luber B, et al. Germline DNA-repair Gene Mutations and Outcomes in Men with Metastatic Castration-resistant Prostate Cancer Receiving First-line Abiraterone and Enzalutamide. Eur Urol 2018;74:218-25. [Crossref] [PubMed]
- Messina C, Cattrini C, Soldato D, et al. BRCA Mutations in Prostate Cancer: Prognostic and Predictive Implications. J Oncol 2020;2020:4986365. [Crossref] [PubMed]
- Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005;434:917-21. [Crossref] [PubMed]
- Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005;434:913-7. [Crossref] [PubMed]
- Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 2014;740:364-78. [Crossref] [PubMed]
- Loriot Y, Massard C, Gross-Goupil M, et al. Combining carboplatin and etoposide in docetaxel-pretreated patients with castration-resistant prostate cancer: a prospective study evaluating also neuroendocrine features. Ann Oncol 2009;20:703-8. [Crossref] [PubMed]
- Hager S, Ackermann CJ, Joerger M, et al. Anti-tumour activity of platinum compounds in advanced prostate cancer-a systematic literature review. Ann Oncol 2016;27:975-84. [Crossref] [PubMed]
- Yap TA, Carden CP, Kaye SB. Beyond chemotherapy: targeted therapies in ovarian cancer. Nat Rev Cancer 2009;9:167-81. [Crossref] [PubMed]
- Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer 2003;3:502-16. [Crossref] [PubMed]
- Ashworth A, Lord CJ. Synthetic lethal therapies for cancer: what's next after PARP inhibitors? Nat Rev Clin Oncol 2018;15:564-76. [Crossref] [PubMed]
- Chernikova SB, Game JC, Brown JM. Inhibiting homologous recombination for cancer therapy. Cancer Biol Ther 2012;13:61-8. [Crossref] [PubMed]
- Gudmundsdottir K, Ashworth A. The roles of BRCA1 and BRCA2 and associated proteins in the maintenance of genomic stability. Oncogene 2006;25:5864-74. [Crossref] [PubMed]
- Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol 2014;15:852-61. [Crossref] [PubMed]
- Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 2015;33:244-50. [Crossref] [PubMed]
- Banerjee S, Moore KN, Colombo N, et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2021;22:1721-31. [Crossref] [PubMed]
- Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N Engl J Med 2016;375:2154-64. [Crossref] [PubMed]
- Coleman RL, Oza AM, Lorusso D, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390:1949-61. [Crossref] [PubMed]
- Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:1274-84. [Crossref] [PubMed]
- Kristeleit R, Shapiro GI, Burris HA, et al. A Phase I-II Study of the Oral PARP Inhibitor Rucaparib in Patients with Germline BRCA1/2-Mutated Ovarian Carcinoma or Other Solid Tumors. Clin Cancer Res 2017;23:4095-106. [Crossref] [PubMed]
- Swisher EM, Lin KK, Oza AM, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol 2017;18:75-87. [Crossref] [PubMed]
- Oza AM, Tinker AV, Oaknin A, et al. Antitumor activity and safety of the PARP inhibitor rucaparib in patients with high-grade ovarian carcinoma and a germline or somatic BRCA1 or BRCA2 mutation: Integrated analysis of data from Study 10 and ARIEL2. Gynecol Oncol 2017;147:267-75. [Crossref] [PubMed]
- González-Martín A, Pothuri B, Vergote I, et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med 2019;381:2391-402. [Crossref] [PubMed]
- Fong PC, Yap TA, Boss DS, et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol 2010;28:2512-9. [Crossref] [PubMed]
- Moore KN, Secord AA, Geller MA, et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol 2019;20:636-48. Erratum in: Lancet Oncol 2019;20:e242. [Crossref] [PubMed]
- Gelmon KA, Tischkowitz M, Mackay H, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol 2011;12:852-61. [Crossref] [PubMed]
- Murai J, Huang SY, Das BB, et al. Trapping of PARP1 and PARP2 by Clinical PARP Inhibitors. Cancer Res 2012;72:5588-99. [Crossref] [PubMed]
- Dedes KJ, Wetterskog D, Mendes-Pereira AM, et al. PTEN deficiency in endometrioid endometrial adenocarcinomas predicts sensitivity to PARP inhibitors. Sci Transl Med 2010;2:53ra75. [Crossref] [PubMed]
- Dinkic C, Jahn F, Zygmunt M, et al. PARP inhibition sensitizes endometrial cancer cells to paclitaxel-induced apoptosis. Oncol Lett 2017;13:2847-51. [Crossref] [PubMed]
- Philip CA, Laskov I, Beauchamp MC, et al. Inhibition of PI3K-AKT-mTOR pathway sensitizes endometrial cancer cell lines to PARP inhibitors. BMC Cancer 2017;17:638. [Crossref] [PubMed]
- Janzen DM, Paik DY, Rosales MA, et al. Low levels of circulating estrogen sensitize PTEN-null endometrial tumors to PARP inhibition in vivo. Mol Cancer Ther 2013;12:2917-28. [Crossref] [PubMed]
- Forster MD, Dedes KJ, Sandhu S, et al. Treatment with olaparib in a patient with PTEN-deficient endometrioid endometrial cancer. Nat Rev Clin Oncol 2011;8:302-6. [Crossref] [PubMed]
- Gockley AA, Kolin DL, Awtrey CS, et al. Durable response in a woman with recurrent low-grade endometrioid endometrial cancer and a germline BRCA2 mutation treated with a PARP inhibitor. Gynecol Oncol 2018;150:219-26. [Crossref] [PubMed]
- Robson M, Im SA, Senkus E, et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N Engl J Med 2017;377:523-33. [Crossref] [PubMed]
- Litton JK, Rugo HS, Ettl J, et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N Engl J Med 2018;379:753-63. [Crossref] [PubMed]
- de Bono J, Ramanathan RK, Mina L, et al. Phase I, Dose-Escalation, Two-Part Trial of the PARP Inhibitor Talazoparib in Patients with Advanced Germline BRCA1/2 Mutations and Selected Sporadic Cancers. Cancer Discov 2017;7:620-9. [Crossref] [PubMed]
- Dhawan MS, Bartelink IH, Aggarwal RR, et al. Differential Toxicity in Patients with and without DNA Repair Mutations: Phase I Study of Carboplatin and Talazoparib in Advanced Solid Tumors. Clin Cancer Res 2017;23:6400-10. [Crossref] [PubMed]
- Boussios S, Karihtala P, Moschetta M, et al. Combined Strategies with Poly (ADP-Ribose) Polymerase (PARP) Inhibitors for the Treatment of Ovarian Cancer: A Literature Review. Diagnostics (Basel) 2019;9:87. [Crossref] [PubMed]
- Domchek SM, Postel-Vinay S, Im SA, et al. Olaparib and durvalumab in patients with germline BRCA-mutated metastatic breast cancer (MEDIOLA): an open-label, multicentre, phase 1/2, basket study. Lancet Oncol 2020;21:1155-64. [Crossref] [PubMed]
- Lee JM, Cimino-Mathews A, Peer CJ, et al. Safety and Clinical Activity of the Programmed Death-Ligand 1 Inhibitor Durvalumab in Combination With Poly (ADP-Ribose) Polymerase Inhibitor Olaparib or Vascular Endothelial Growth Factor Receptor 1-3 Inhibitor Cediranib in Women's Cancers: A Dose-Escalation, Phase I Study. J Clin Oncol 2017;35:2193-202. [Crossref] [PubMed]
- Konstantinopoulos PA, Waggoner SE, Vidal GA, et al. TOPACIO/Keynote-162 (NCT02657889): A phase 1/2 study of niraparib + pembrolizumab in patients (pts) with advanced triple-negative breast cancer or recurrent ovarian cancer (ROC)—Results from ROC cohort. J Clin Oncol 2018;36:106. [Crossref]
- Liu JF, Barry WT, Birrer M, et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised phase 2 study. Lancet Oncol 2014;15:1207-14. [Crossref] [PubMed]
- Boussios S, Abson C, Moschetta M, et al. Poly (ADP-Ribose) Polymerase Inhibitors: Talazoparib in Ovarian Cancer and Beyond. Drugs R D 2020;20:55-73. [Crossref] [PubMed]
- Turner N, Tutt A, Ashworth A. Hallmarks of 'BRCAness' in sporadic cancers. Nat Rev Cancer 2004;4:814-9. [Crossref] [PubMed]
- Lord CJ, Ashworth A. BRCAness revisited. Nat Rev Cancer 2016;16:110-20. [Crossref] [PubMed]
- Kondrashova O, Scott CL. Clarifying the role of EMSY in DNA repair in ovarian cancer. Cancer 2019;125:2720-4. [Crossref] [PubMed]
- Davies H, Glodzik D, Morganella S, et al. HRDetect is a predictor of BRCA1 and BRCA2 deficiency based on mutational signatures. Nat Med 2017;23:517-25. [Crossref] [PubMed]
- Ray-Coquard I, Pautier P, Pignata S, et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N Engl J Med 2019;381:2416-28. [Crossref] [PubMed]
- de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med 2020;382:2091-102. [Crossref] [PubMed]
- Marcotte R, Brown KR, Suarez F, et al. Essential gene profiles in breast, pancreatic, and ovarian cancer cells. Cancer Discov 2012;2:172-89. [Crossref] [PubMed]
- Kampmann M. CRISPRi and CRISPRa Screens in Mammalian Cells for Precision Biology and Medicine. ACS Chem Biol 2018;13:406-16. [Crossref] [PubMed]
- Dixit A, Parnas O, Li B, et al. Perturb-Seq: Dissecting Molecular Circuits with Scalable Single-Cell RNA Profiling of Pooled Genetic Screens. Cell 2016;167:1853-1866.e17. [Crossref] [PubMed]
- Boussios S, Moschetta M, Karihtala P, et al. Development of new poly(ADP-ribose) polymerase (PARP) inhibitors in ovarian cancer: Quo Vadis? Ann Transl Med 2020;8:1706. [Crossref] [PubMed]
- Gogola E, Duarte AA, de Ruiter JR, et al. Selective Loss of PARG Restores PARylation and Counteracts PARP Inhibitor-Mediated Synthetic Lethality. Cancer Cell 2018;33:1078-1093.e12. [Crossref] [PubMed]
- Bunting SF, Callén E, Wong N, et al. 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell 2010;141:243-54. [Crossref] [PubMed]
- Johnson SF, Cruz C, Greifenberg AK, et al. CDK12 Inhibition Reverses De Novo and Acquired PARP Inhibitor Resistance in BRCA Wild-Type and Mutated Models of Triple-Negative Breast Cancer. Cell Rep 2016;17:2367-81. [Crossref] [PubMed]
- Garcia TB, Snedeker JC, Baturin D, et al. A Small-Molecule Inhibitor of WEE1, AZD1775, Synergizes with Olaparib by Impairing Homologous Recombination and Enhancing DNA Damage and Apoptosis in Acute Leukemia. Mol Cancer Ther 2017;16:2058-68. [Crossref] [PubMed]
- Kim H, Xu H, George E, et al. Combining PARP with ATR inhibition overcomes PARP inhibitor and platinum resistance in ovarian cancer models. Nat Commun 2020;11:3726. [Crossref] [PubMed]
Cite this article as: Chebly A, Yammine T, Rassy E, Boussios S, Moschetta M, Farra C. Current evidence of BRCA mutations in genitourinary and gynecologic tumors: a scoping review. Precis Cancer Med 2022;5:17.

