ALK non-small cell lung cancer sequence of treatment: a case report
Introduction: “ALK sequence treatment for NSCLC”
Lung cancer is one of the most frequent causes of cancer-related mortality, and most patients have metastases in the early stages of the disease (1). Treatment for these patients has historically consisted of systemic chemotherapy. Knowledge of the molecular pathways involved in metastatic non-small cell lung cancer (NSCLC) can improve our understanding and enable the development of target-specific agents in several subtypes of lung adenocarcinoma (2).
One of the molecular pathways involves rearrangement of the anaplastic lymphoma kinase (ALK) gene. This gene alteration has been reported in 2–7% (3) of patients with NSCLC, and the most common alteration of ALK is its fusion with echinoderm microtubule-associated protein-like 4 (EML4). This led to the development of specific molecular treatments for these patients, who are usually nonsmokers or light smokers and are younger at onset, such as children and young adults, than those patients who do not have this molecular alteration. Patients with NSCLC-positive ALK-EML4 gene fusion have a better response to ALK inhibitors than other standard treatments (4).
The first of these ALK inhibitors was crizotinib, which showed an improvement in overall survival (OS) in patients treated first with crizotinib and then an ALK tyrosine kinase inhibitor and adequate safety. In the final OS analysis of the PROFILE 1014 study, these patients had a median OS that was not reached (5). The benefit in OS after treatment with an ALK inhibitor first and then others at disease progression has been shown in other studies, as described here and again later. In the updated OS ALEX study, the median OS was 57.4 months in the crizotinib arm versus not reached in the alectinib arm, possibly due to access to pemetrexed-based chemotherapy and other ALK tyrosine kinase inhibitors. When we analysed real-world data (RWD), we found similar results after progression from first TKI and subsequent treatment with other TKIs; the median OS was 89.6 months, which was statistically significant from the diagnosis of metastatic disease in the CLINALK study, and the median OS was 90.3±24.4 months in the GLASS study, which was not statistically significant in either cohort (patients ALK+ and ROS+).
The patient described in the case report herein achieved prolonged OS. The sequence of treatment is increasingly important in these patients with progress in genomic studies and resistance mechanisms.
Case presentation
We report a case of a 52-year-old woman with a previous tobacco history of 13 pack-years. In May 2010, she was evaluated due to cough and dyspnoea. She underwent a chest RX, CT scan, PET-TC scan and bronchoscopy and received a final diagnosis of locally advanced lung adenocarcinoma, stage IIIB (right upper lobe mass and ipsilateral and subcarinal lymphadenopathy). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
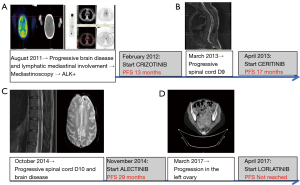
In June 2010, she received curative-intent concurrent chemoradiation (cisplatin/paclitaxel and radiotherapy, total dose 69.6 Gy) and achieved a partial response, but brain progression was observed in October 2010 (three new nodules located in the left temporal lobe, left frontal lobe and right corpus callosum). Then, she received radiosurgery with CyberKnife in all lesions with an adequate brain disease response followed by six cycles of carboplatin/pemetrexed and maintenance with pemetrexed for 9 months. In August 2011, a PET-CT scan showed new lymphatic mediastinal involvement and brain disease under progression. Whole-brain radiotherapy was performed, and mediastinoscopy showed a mutation in ALK (Figure 1A).
In February 2012, crizotinib was started, and the patient achieved a good partial response and adequate tolerance for 13 months. In March 2013, she developed progressive bone disease with D9 cord compression (Figure 1B). She received radiotherapy (20 Gy) and developed residual paraplegia.
From April 2013 to September 2014, she started treatment with ceritinib in the ASCEND-5 clinical trial, with poor tolerance and grade 3 hepatotoxicity, which required two dose reductions of ceritinib. With this treatment, she achieved stable disease for 17 months.
In October 2014, images showed a new brain and bone disease (Figure 1C). Alectinib was started, and the patient exhibited a partial response and excellent tolerance without toxicities. The PFS on this second-generation ALK inhibitor was 29 months.
Finally, during alectinib treatment in March 2017, a CT scan revealed a new mass in the left ovary and lymph node involvement (Figure 1D). Radical gynaecological surgery was performed with a final pathological report consistent with metastasis in the left ovary of lung cancer adenocarcinoma with a new EML4-ALK (variant 3a/b) G1202R mutation detected by NGS (Foundation One). In this patient, the tumour mutation burden was low (2 Mut/Mb), and MLL3 I4835Fs*4 was detected. At this time, she started treatment with lorlatinib, which is currently ongoing and has adequate tolerance. The only side effects are occasional diplopia and hypercholesterolemia and hypertriglyceridaemia, which are well controlled with treatment.
The current OS of our patient has been prolonged for more than 10 years with the optimal sequencing of next-generation ALK TKIs.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
All patients with stage IV lung cancer adenocarcinoma should be assessed for several molecular markers, including ALK gene rearrangements. This alteration occurs more frequently in younger patients and nonsmokers. ALK translocations can be identified by next-generation sequencing (NGS) panels, immunohistochemistry (IHC), or fluorescence in situ hybridization (FISH) (6). In metastatic NSCLC, the presence of an ALK gene rearrangement (ALK positivity) strongly predicts sensitivity to ALK inhibitors, so this treatment is the best option to prolong progression-free survival (PFS) in these patients (7). Several clinical trials have shown the superiority of ALK inhibitors.
The first ALK inhibitor that showed superiority over chemotherapy was crizotinib, both in front-line and subsequent-line treatments. In our case report, our patient was diagnosed with ALK-positive lung cancer adenocarcinoma when she was receiving first-line metastatic disease treatment with chemotherapy. The second line of treatment was crizotinib. In the Clinical Trial PROFILE 1014, crizotinib was shown to be superior to chemotherapy. The primary endpoint was PFS. With a follow-up of 17 months, the ORR with crizotinib was higher than that with chemotherapy (74% versus 45%), and the median PFS was prolonged (10.9 versus 7 months; HR 0.45) (8). In the ASCEND-4 study that compared second-generation ALK inhibitors versus chemotherapy, ceritinib showed a median PFS of 16.6 months versus 8.1 months in the chemotherapy arm with an HR of 95% CI: 0.42 to 0.73, P<0.001, with no statistically significant differences in OS between the two arms (9). In the global ALEX study, patients were randomly assigned to first-line alectinib versus crizotinib. Patients receiving alectinib had a 53% reduction in the risk of progression or death (HR 0.47, 95% CI: 0.34–0.65). After an additional 10 months of follow-up in an update to the global ALEX study, the median PFS was 35 months in the alectinib group versus 11 months in the crizotinib group (HR 0.43). OS results are not yet mature (10). Lorlatinib is a powerful third-generation ALK TKI that has been approved for the treatment of patients with ALK-positive NSCLC who have progressed on alectinib or ceritinib as front-line ALK inhibitor therapy or crizotinib and at least one other ALK inhibitor. Because lorlatinib has activity against all of the known ALK inhibitor resistance mutations, including G1202R which was detected in our patient, it is the preferred agent in cases of alectinib resistance (11). This mutation confers resistance to other next-generation ALK inhibitors, including ceritinib, alectinib and brigatinib (12). FDA approval for lorlatinib is based on the results of a phase II study (B7461001) (13). The overall response rate with lorlatinib among these patients was 47% (partial response 45% and complete response 2%). Efficacy according to prior treatment was as follows: post crizotinib: ORR 73%, median PFS 11.1 months, and median duration of response not reached; after one or more second-generation ALK inhibitors: ORR 40%, median PFS 6.9 months, and median duration of response 7.1 months (14).
The updated clinical trial PROFILE 1014 was recently published. With a median follow-up duration of 46 months, the final analysis of this clinical trial showed that the median OS with chemotherapy was 47.5 months versus not reached with crizotinib. The OS rates at different time points (1 year, 18 months and 4 years) were higher with crizotinib than with chemotherapy (83.5% vs. 78.4%, 71.5% vs. 66.6% and 56.6% vs. 49.1%, respectively). The hazard ratio (HR) was better with crizotinib (HR 0.346; 95% bootstrap CI: 0.081 to 0.718), with a median OS of 59.8 months with this treatment (95% CI: 46.6 months to NR) and 19.2 months with chemotherapy (95% CI: 13.6 months to NR) when OS was adjusted for crossover. Crizotinib has a significant effect on PFS. However, the absence of a statistically significant difference in OS may be due to the impact of effective post-progression therapy on the outcome and crossover (5). Depending on the type of subsequent treatment received, OS was also analysed in four subgroups. In the group who received crizotinib and at least one ALK inhibitor in any line of subsequent treatment, the results showed the longest OS, with the median OS not reached in these patients. Our case report shows that our patient benefited from the sequence of ALK inhibitors described herein, with an OS duration of more than 59.8 months. In a retrospective study in which patients received platinum pemetrexed-based chemotherapy after progression following one or more second-generation TKIs, there was an objective response rate (29.7%) with this type of chemotherapy with a median duration of response of 6.4 months (15).
When we analyse real-world databases, the benefit is similar to that of clinical trials. In the French CLINALK study, OS was analysed in patients with ALK-positive NSCLC who had received crizotinib. After progression on crizotinib, the patients who received next-generation ALK inhibitors versus subsequent drugs other than next-generation ALK inhibitors or best supportive care had better OS: 89.6 months from the diagnosis of metastatic disease and a median post-progression disease survival of 25 months (16). In the GLASS retrospective study, the disease control rate in ALK+ patients was 91% with lorlatinib, with objective response rates of 60% and 62% (extracranial and intracranial disease, respectively). The median OS was 90.3±24.4 months, which was not statistically significant (17). In another retrospective study of real-world data in Canada, ALK+ patients who received crizotinib and alectinib had median PFS and OS of 17 months and 48.5 months, respectively (18).
In NSCLC, brain disease is associated with poor prognosis, but in patients with an ALK mutation, the response to these treatments is better than that in patients without an ALK mutation. The ALK inhibitors that have shown more efficacy in brain disease are alectinib and lorlatinib. Alectinib showed a PFS benefit over crizotinib irrespective of the presence or absence of CNS metastases at baseline. For patients who had CNS metastases at the beginning of treatment, the median PFS was higher with alectinib (25.4 months; 95% CI: 9.2 months–NE months) than with crizotinib (7.4 months; 95% CI: 6.6–9.6 months). The median PFS was 38.6 months with alectinib and 14.8 months with crizotinib (HR 0.46, 95% CI: 0.31–0.68) for patients without baseline CNS metastases. Our patient, who had brain metastases at baseline and at the fifth line of treatment, had a PFS duration of 29 months with alectinib, which is longer than that reported in the global ALEX study. In the phase II study (B7461001), lorlatinib patients were analysed if they had progressive disease and were divided into non-CNS or CNS progression groups. At 12 months, the cumulative incidence rate (CIR) was higher in patients without CNS progression who received crizotinib as their first treatment without baseline CNS metastases than in patients with CNS progression (43% vs. 9%, respectively). In the patients with baseline CNS metastases, the CIR was the same (22% for both groups). At 12 months in patients who had been treated with ≥1 second-generation ALK TKI, the CIR of non-CNS progression was higher than that of CNS progression in patients both with and without baseline CNS metastases (35% vs. 23% and 55% vs. 12%, respectively) (19,20).
In pretreated patients with ALK-positive NSCLC with or without baseline CNS metastases and who experienced disease progression on crizotinib or second-generation ALK TKIs, lorlatinib had intracranial activity. PFS on lorlatinib in our patient was not reached because the patient is still on this treatment. Recently, interim analysis data from the Crown Clinical Trial have been published. It is a phase 3 trial in patients with ALK-positive NSCLC metastatic disease comparing lorlatinib versus crizotinib as the first treatment. The PFS and the intracranial response were better in those patients who were treated with lorlatinib as the first option of treatment. The percentage of progression-free survival at 12 months was 39% in the crizotinib arm (95% CI: 30 to 48) and 78% in the lorlatinib group (95% CI: 70 to 84), with an HR of 0.28, P<0.001, by independent central review. The median PFS at the cut-off data was 9.3 months in the crizotinib arm versus not reached in the lorlatinib arm. In those patients with all types of CNS metastases at baseline, the intracranial response was higher with lorlatinib, 66% (95% CI: 49% to 80%) versus 20% (95% CI: 9% to 36%) in the crizotinib arm. In measurable CNS metastases the intracranial response was 23% in the crizotinib arm versus 82% in the lorlatinib arm. At 12 months the duration of intracranial response was 0% in the crizotinib arm versus 72% in the lorlatinib arm and the incidence of CNS metastases as the first event was 33% versus 3% in the crizotinib group versus lorlatinib group, respectively, with a HR 0.06; 95% CI: 0.02 to 0.18. The most common adverse events were hypercholesterolemia and hyperlipidaemia, peripheral neuropathy, oedema and cognitive effects. These data make it necessary for other clinical trials to address second-generation to third-generation ALK inhibitors to better define the first line of treatment in our patients (21,22).
Although alectinib is currently the standard first-line treatment in these patients, in this case report, we propose that receiving sequential ALK inhibitors may be a treatment strategy to improve OS in these patients. Resistance mechanisms to ALK inhibitors necessitate new diagnostic strategies to provide patients with the most effective therapy. In ALK-rearranged NSCLC patients who relapse on next-generation ALK-TKIs, single circulating tumour cell (CTC) sequencing can be used to identify secondary resistance mutation mechanisms (23). In patients treated with second- and third-generation ALK TKIs, another study analysed plasma specimens using a NGS platform. With each successive generation of ALK TKIs, ALK resistance mutations increase and may be underestimated by tumour genotyping (24). Patient-derived models combined with longitudinal tumour sampling can also contribute to knowledge on tumour dynamics and biological processes underlying disease progression. It is important to determine the best sequence of next-generation ALK-TKIs for the treatment of our patients (25).
Greater molecular knowledge of this type of tumour and its secondary resistance mutations is needed to define the optimal treatment for each patient with ALK-rearranged NSCLC, making it possible to delay chemotherapy in these patients by improving their quality of life and tolerance to treatments.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by Guest Editors (Jesús Corral, Laura Mezquita and Ernest Nadal) for the series “ALK and ROS-1 NSCLC Patients Treatment Approach Based on Genomic Profile by Liquid Biopsy” published in Precision Cancer Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-13/coif). The series “ALK and ROS-1 NSCLC Patients Treatment Approach Based on Genomic Profile by Liquid Biopsy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: All authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [Crossref] [PubMed]
- Pikor LA, Ramnarine VR, Lam S, et al. Genetic alterations defining NSCLC subtypes and their therapeutic implications. Lung Cancer 2013;82:179-89. [Crossref] [PubMed]
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [Crossref] [PubMed]
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. [Crossref] [PubMed]
- Solomon BJ, Kim DW, Wu YL, et al. Final Overall Survival Analysis From a Study Comparing First-Line Crizotinib Versus Chemotherapy in ALK-Mutation-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:2251-8. [Crossref] [PubMed]
- Lin C, Shi X, Yang S, et al. Comparison of ALK detection by FISH, IHC and NGS to predict benefit from crizotinib in advanced non-small-cell lung cancer. Lung Cancer 2019;131:62-8. [Crossref] [PubMed]
- Koivunen JP, Mermel C, Zejnullahu K, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res 2008;14:4275-83. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389:917-29. Erratum in: Lancet 2017;389:908. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Camidge DR, Dziadziuszko R, Peters S, et al. Updated Efficacy and Safety Data and Impact of the EML4-ALK Fusion Variant on the Efficacy of Alectinib in Untreated ALK-Positive Advanced Non-Small Cell Lung Cancer in the Global Phase III ALEX Study. J Thorac Oncol 2019;14:1233-43. [Crossref] [PubMed]
- Baglivo S, Ricciuti B, Ludovini V, et al. Dramatic Response to Lorlatinib in a Heavily Pretreated Lung Adenocarcinoma Patient Harboring G1202R Mutation and a Synchronous Novel R1192P ALK Point Mutation. J Thorac Oncol 2018;13:e145-7. [Crossref] [PubMed]
- Gainor JF, Dardaei L, Yoda S, et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov 2016;6:1118-33. [Crossref] [PubMed]
- Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol 2018;19:1654-67. [Crossref] [PubMed]
- Lin JJ, Schoenfeld AJ, Zhu VW, et al. Efficacy of Platinum/Pemetrexed Combination Chemotherapy in ALK-Positive NSCLC Refractory to Second-Generation ALK Inhibitors. J Thorac Oncol 2020;15:258-65. [Crossref] [PubMed]
- Duruisseaux M, Besse B, Cadranel J, et al. Overall survival with crizotinib and next-generation ALK inhibitors in ALK-positive non-small-cell lung cancer (IFCT-1302 CLINALK): a French nationwide cohort retrospective study. Oncotarget 2017;8:21903-17. [Crossref] [PubMed]
- Peled N, Gillis R, Kilick S, et al. GLASS: Global Lorlatinib for ALK(+) and ROS1(+) retrospective Study: real world data of 123 NSCLC patients. Lung Cancer 2020;148:48-54. [Crossref] [PubMed]
- Gibson AJW, Box A, Dean ML, et al. Retrospective Real-World Outcomes for Patients With ALK-Rearranged Lung Cancer Receiving ALK Receptor Tyrosine Kinase Inhibitors. JTO Clin Res Rep 021;2:100157.
- Mok T, Camidge DR, Gadgeel SM, et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol 2020;31:1056-64. [Crossref] [PubMed]
- Bauer TM, Shaw AT, Johnson ML, et al. Brain Penetration of Lorlatinib: Cumulative Incidences of CNS and Non-CNS Progression with Lorlatinib in Patients with Previously Treated ALK-Positive Non-Small-Cell Lung Cancer. Target Oncol 2020;15:55-65. [Crossref] [PubMed]
- Shaw AT, Solomon BJ, Besse B, et al. ALK Resistance Mutations and Efficacy of Lorlatinib in Advanced Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2019;37:1370-9. [Crossref] [PubMed]
- Shaw AT, Bauer TM, de Marinis F, et al. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N Engl J Med 2020;383:2018-29. [Crossref] [PubMed]
- Pailler E, Faugeroux V, Oulhen M, et al. Acquired Resistance Mutations to ALK Inhibitors Identified by Single Circulating Tumor Cell Sequencing in ALK-Rearranged Non-Small-Cell Lung Cancer. Clin Cancer Res 2019;25:6671-82. [Crossref] [PubMed]
- Dagogo-Jack I, Rooney M, Lin JJ, et al. Treatment with Next-Generation ALK Inhibitors Fuels Plasma ALK Mutation Diversity. Clin Cancer Res 2019;25:6662-70. [Crossref] [PubMed]
- Recondo G, Mezquita L, Facchinetti F, et al. Diverse Resistance Mechanisms to the Third-Generation ALK Inhibitor Lorlatinib in ALK-Rearranged Lung Cancer. Clin Cancer Res 2020;26:242-55. [Crossref] [PubMed]
Cite this article as: Miriam AG, Amparo SG, Reyes BC, Cristina BPJ, Irene CG. ALK non-small cell lung cancer sequence of treatment: a case report. Precis Cancer Med 2022;5:8.