Wielding a double-edged sword in head and neck cancers—diagnosis, risk factors and mitigation strategies of radiation-induced head and neck sarcomas: a narrative review
Introduction
Radiotherapy (RT) is an essential arm in the contemporary multimodality management of head and neck cancers. The use of both definitive and adjuvant RT, in addition to improvements in surgery and systemic therapy, have led to an increase in survival of patients over time (1).
Delayed complications are the perils of our own success, which only occur in patients who have managed to overcome the first diagnosis. Hall, during his inaugural Frank-Ellis lecture said “There can be few worse things for a patient than to survive the initial treatment, live with the long-term morbidity of treatment, only to find that they have developed a radiation-induced second cancer, which may have a worse prognosis than their original tumour.” (2).
Radiation-induced sarcoma (RIS) is a rare occurrence with estimated risks of 0.1% to 0.3% (3-5). Amongst all sarcomas, RIS accounts for only 3% to 6% of cases (6,7). It is undoubtedly challenging to differentiate RIS from de novo sarcoma. Cahan proposed a diagnostic criterion, which was later modified by Murray (8,9). These are as follows:
- Tumour arises in a field that has been previously irradiated.
- First tumour is histologically distinct from the subsequent one.
- No evidence of the new tumour at the time of the initial RT.
- New tumour developed after a latency period from RT.
Radiation-induced head and neck sarcoma (RISHNN) represents merely 1% of all head and neck sarcomas. Although rare, RISHNN poses as a challenging entity to diagnose and manage. Patients have a poorer prognosis than de novo sarcomas, with the 5 year overall survival (OS) rate ranging between 24–38% (5,10-12). There is no specific area within the head and neck region which is predisposed to developing RISHNN. Studies from Asia seem to indicate that the para-nasal sinuses are most commonly affected (13,14). However, this may be related to the endemic nature of nasopharyngeal carcinoma within Asia. This condition is almost exclusively treated with chemo-RT, with the para-nasal sinuses being irradiated electively.
Patients with RISHNN often experience a delay in diagnosis because of the challenges in examining previously radiated tissue, lack of specific symptoms, long latency period and difficulties in obtaining histopathological confirmation. As the treatment options for RISHNN are limited, mitigation strategies should be considered upfront for patients considered to be at higher risk of RISHNN.
Therefore, this narrative review aims to present a summary of (I) the incidence, (II) diagnosis, (III) contributing risk factors, (IV) management options, (V) prognosis and lastly, (VI) mitigation strategies.
Methods
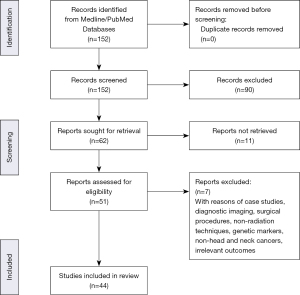
Literature search was performed using the MEDLINE/PubMed database. MeSH headings were used to identify articles pertaining to RISHNN. The headings include “radiation – induced”, “sarcomas”, “head and neck sarcomas” and “radiation therapy”. Articles published between years 1990 to 2021 were reviewed. Non-English language articles were excluded. Non radiation induced head and neck sarcomas were excluded. Figure 1 presents the PRISMA flow diagram summarizing the search strategy and the process of study selection. We present the following article in accordance with the Narrative Review reporting checklist (available at https://dx.doi.org/10.21037/pcm-21-30).
Incidence
Multiple studies have reported the cumulative incidence of developing RIS to range between 0.03% to 0.3% (4,15,16). The length of follow-up is important to consider when interpreting this, as the median time to developing RIS is ~15 years (12). Yap et al. reported the cumulative incidence of sarcoma in breast cancer patients who received RT to be 0.32% at 15 years, compared to 0.23% in the patients who didn’t receive RT (P=0.001) (17). Although the magnitude of the incremental risk is significant, the absolute risk is low.
As mentioned earlier, it does appear that the incidence of RIS is increasing over the years. This is exemplified by the cohort data from the Norway Cancer registry, spanning from years 1960–2007 (18). The mean incidence of secondary sarcoma (not exclusive to RIS) increased from 2.6% in 1960s to 14% from years 2000–2007. This incremental change trumps the increase of de novo sarcoma and cancers in general, with a larger average annual percent change seen in secondary sarcomas (6.2 vs. 2.5).
This increase could be attributed to several factors (19). Firstly, new surgical techniques, systemic therapies and other treatment options confer better survival outcomes for patients receiving RT (20). Secondly, the use of RT as part of cancer treatment regime has been steadily increasing. Lastly, although modern RT techniques with intensity modulation allow for better normal tissue sparing, this is at the expense of larger volumes of normal tissues receiving low dose radiation. As even exposure to low dose radiation may result in genomic instability facilitating malignant change (21).
Within the context of RISHNN, Coca-Pelaz et al. performed a recent systematic review of the literature spanning from years 2000 to 2020 (22). The frequency of RISHNN was 0.15%, with the most common site of primary radiation being the nasopharynx. The mean latency between primary radiation and RISHNN was 11.1 years (range, 1.3–38 years). The most common site of RISHNN involves the sino-nasal region. The most common histological subtypes were osteosarcoma and fibrosarcoma.
Diagnosis
Imaging
Imaging plays an important role in the diagnosis and workup of RISHNN. Computed tomography (CT) and MRI play a complementary role, with MRI providing better soft tissue definition. They both provide multi-planar cross-sectional information on the locoregional tumour involvement, tissue composition and aid in planning biopsies for histopathological confirmation (10,23). In a study involving 63 patients, cross sectional imaging findings of bone sarcomas included soft tissue mass, cortical bone destruction, tumour mineralisation and periosteal reaction (24). In contrast, soft tissue sarcomas were commonly associated with findings of a destructive soft tissue mass in the absence of bone expansion and periosteal reaction.
Often, one is unable to distinguish RISHNN from de novo sarcomas based on imaging alone. However, MRI finding of normal marrow replacement with fat is suggestive of previous radiation (i.e., bright signal on T1-weighted imaging). This leans towards RISHNN (25).
In the workup for metastatic deposits, which typically involve the lung and/or liver, practice guidelines from the National Comprehensive Cancer Network (NCCN) recommend a FDG-PET/CT scan (26,27).
Histopathological confirmation
Biopsy of the suspected primary lesion, or metastatic deposit, is crucial for diagnosis and treatment. Sole reliance on imaging is insufficient to ascertain between recurrence of the primary tumour, treatment-related changes or the development of a secondary malignancy. For both bone and soft tissue sarcomas, a core needle biopsy is minimally required (28). The placement and trajectory of the biopsy must be carefully planned, often in consultation with the sarcoma surgeon, in order not to compromise future surgical plans.
Histopathological findings
The histopathologic spectrum of RIS is broad and although all subtypes were observed in RIS, their frequency of occurrence varies from those of de novo sarcomas (29,30). In the review by Zhu et al. (n=323), the most common subtype was osteosarcoma (34.1%) followed by fibrosarcoma (19.2%) and unidentate pleomorphic sarcoma (15.8%) (31). Although the median size of RIS tumours are comparable to that of de novo sarcomas, RIS tend to have a higher proportion of high-grade tumors, with the presence of tumour necrosis (6,18,29,32). While there is still no pathogenomic features to discern between RIS and de novo sarcomas arising within previously irradiated fields, morphological changes of adjacent tissues may provide guidance if they demonstrate radiation-related changes such as atypical fibroblasts, dense cellular fibrosis, distortions in vascular structures (33).
Molecular signatures
Clearly, there are limitations in distinguishing RIS from de novo sarcoma based on clinical suspicion, imaging and histopathology alone. Therefore, the use of molecular and genetic signatures have been of interest in recent years (34).
Panse et al. found a complete loss of histone H3 lysine 27 trimethylation (H3K27me3) in 19% of patients with RIS in two tertiary care institutions (35). This was present in radiation-associated sarcomas of varying histological subtypes. In a study comparing MYC amplifications between RIS and de novo sarcomas, a significantly higher number of MYC amplifications were found in RIS than in de novo sarcomas (P<0.0001) (36). This has been found for leiomyosarcomas, undifferentiated pleomorphic sarcomas subtypes and is especially pronounced in angiosarcoma. However, Mito and colleagues cautioned that MYC expression is generally uncommon amongst RIS (save for angiosarcoma), and has a similar prevalence in de novo sarcomas. Therefore, it has limited diagnostic value in situations other than radiation-induced angiosarcomas (37). A study comparing the transcriptome of RI angiosarcoma with that of de novo angiosarcoma found 135 gene signatures, indicating mitochondrial dysfunction with chronic oxidative stress. This may aid in the diagnosis of RIS (38,39).
At present, molecular or genetic signatures influencing the diagnosis of RIS is still in its infancy. Although the presence of the above information may increase the probability of RIS (in addition to Cahan’s criteria), there remains no gold standard within clinical practice (8,40).
Contributory risk factors
The four main factors that increase the risks of developing RISHNN cancers are: (I) age at first radiation exposure; (II) radiation-related factors; (III) previous cytotoxic chemotherapy; (IV) inherent genetic predisposition.
Age at first radiation exposure
The younger the age at first radiation exposure, the higher the risk for secondary sarcomas (41). Childhood Cancer Survivor Study found that the risk of secondary RIS was more than nine-fold higher amongst childhood cancer survivors compared to the general childhood population. Highest risk was observed in paediatric patients under four years old at the time of initial cancer diagnosis (41). Besides the long latent period, it is also likely that paediatric patients are more sensitive to radiation exposure. Possible explanations include a greater proportion of stem cells (exposed to radiation) in young patients predisposing them to be more prone to sarcoma-genesis. The development of childhood tumours could be related to underlying germline mutations, which predisposes them to RIS (41,42). An example would be retinoblastoma.
Radiation related factors
Dose
Carcinogenesis is a stochastic late effect of radiation exposure, where there is no safety radiation threshold dose below which there is no risk of second malignancy (22,30). Two Japanese studies have reported RIS occurring at doses below 15 Gy with both describing increased risks of sarcogenesis with increasing radiation dose (43,44). The development of sarcoma exhibits a linear dose-response relationship, with soft tissue sarcoma having an excess relative risk of 1.01 per Gy (95% CI: 0.13 to 2.46 Gy; P=0.019) and osteosarcoma which having an excess relative risk of 7.5 per Gy (95% CI: 1.34 to 23.14 per Gy) after exceeding the dose threshold of 0.85 Gy (43,44). This correlation of increased sarcoma-genesis risks with increasing radiation dose is consistent with RIS studies on different body sites studied over the years (3,45-47). It is unclear if the linearity in the dose response can be extrapolated to higher doses. While there is no threshold dose at which RIS occurs, RIS is generally thought to occur at doses that catalyze sublethal damage at the cellular level that eventually results in sarcoma-genesis. Hence, it can be theorized that at extremely high radiation doses, where lethal damage predominates, the correlation between increased sarcoma-genesis risks with increasing radiation dose ceases. However, this theory has been contested by Berrington de Gonzalez et al. who demonstrated that there is little evidence that the dose-response curve plateaus at higher doses (such as >60 Gy) (40).
Despite the varying theories, the prevalence of RIS appearing in areas of intermediate dose areas within the primary radiation field supports the generally accepted theory that radiation dose delivered in the periphery areas surrounding the primary tumour is more important than the radiation dose delivered to the primary tumour (22,48). However, this is not concrete as there is scant published data.
Technique
It has been theorized that the use of newer RT techniques such as intensity-modulated radiation therapy (IMRT) and volumetric modulated arc therapy (VMAT), commonly used in head and neck cancer treatment in the modern era, has resulted in an increase in radiation induced secondary malignancies (30,49). They are an evolvement of three-dimensional conformal radiation therapy (3D CRT) and utilizes multiple modulating fields to create highly conformal doses specific to the tumour shape and volume. This has enabled curative doses delivered to the tumours while simultaneously reducing dose to healthy surrounding organs (50). While IMRT/VMAT is able to produce conformal RT plans for both high and intermediate dose regions (e.g., 80% and 50% respectively), the downside is a larger volume of low dose splash (e.g., 10–20%) (50,51).
Data to support or refute this comes mostly from modelling studies. There is no strong evidence from long term prospective cohort studies. For example, Serizawa and Hall, suggested a two-fold increase in secondary malignancies with the use of IMRT/VMAT, compared to 3DCRT. Similarly, Stathakis et al. showed a 40% increase in second malignancy risk when head and neck cancer patients were treated with IMRT compared to 3DCRT (52). In contrast, data from Gupta et al. did not show any increased risk of secondary malignancy with 3DCRT vs. IMRT. However, this trial only included 60 patients (i.e., likely underpowered for this outcome), with a median follow-up of 10 years (53).
Other factors to consider include beam energy (due to secondary neutron production) and monitor unit usage (resulting in radiation leakage through the machine) (54-57). It also remains unclear if the use of image-guided RT for head and neck cancers contributes to this risk. Performing daily IGRT (cone-beam CT scans) prior to each fraction, may allow a reduction in planning target volume margin. However, each cone-beam CT accounts for ~3–10 cGy to a large volume of tissue (58). Kim et al., based on the linear no threshold model, estimated the lifetime attributable risk for secondary cancer to be ~4% if 30 CBCT scans were performed for pelvic tumours (59). This has to be interpreted cautiously as the risk depends on the chosen model. It is also unclear if it can be extrapolated to the head and neck region.
Previous cytotoxic chemotherapy
Particularly for childhood cancers, exposure to chemotherapy, in addition to RT, has been proven to increase the relative risk of RIS. Noticeably, the use of anthracyclines and alkylating agents have demonstrated strong associations with increased risks (41,42). Two cohort studies investigating the use of chemotherapy with RT for childhood cancers have found a 4 fold increase of RIS risk with cumulative drug exposure (41,42). However, it is still unclear whether these results can be extrapolated to adults.
Inherent genetic predisposition
Double strand breaks induced by primary radiation exposure can result in genomic instability which increases the potential of carcinogenesis and sarcoma-genesis (60). Certain rare familial genetic syndromes such as Li-Fraumei, Retinoblastoma, Neurofibromatosis 1, and Nijmnegen breakage syndrome are associated with a higher risk of RIS. It is undeniable that the baseline risk of developing de novo malignancies is high in these individuals. Exposure to radiation may potentially increase the baseline risk of developing sarcoma. However, clinical data is scant as most of these patients would have been excluded from clinical trials. Also, the incidence of familial genetic syndromes is uncommon. In a large cohort study of retinoblastoma survivors, the use of RT increased the risk of secondary malignancies in both patients with hereditary and non-hereditary forms of retinoblastoma. Similarly, Hisada et al. (61) reported that RT contributed to the already elevated risk of second cancers in patients with familial Li Fraumeni syndrome.
Management options
RISHNN is a rare diagnosis, and as a result, there are no practice guidelines or randomized studies to follow. Moreover, RIS is a mixed bag of conditions involving varying histology subtypes, and affecting various anatomical sites (22,30,62). As such, management options are derived from de novo sarcomas, and from small institutional series of RIS patients. Surgical resection with wide negative margins is the preferred method for patients with non-metastatic RIS, and is the only chance of cure (30,62). In a multi-institutional retrospective study of 80 histologically confirmed sarcomas within previously irradiated fields, the OS rates at 2 years was 69% and 5 years was 39% for patients who had surgery as opposed to 10% and 0% at 2 years and 5 years respectively for those receiving chemotherapy alone (63). A recent systematic review including only patients with RISHNN found that approximately, only half of the patients with resectable tumors underwent surgery (22). In the context of head and neck region, we must acknowledge that this may not be possible due to complex neuro-vascular structures and adjacent vital organs, within the vicinity, which may compromise surgical resection. Moreover, previous radiation induced fibrotic changes in tissues with impaired circulation can lead to increased surgical complications, such as poor wound healing (64). This presents challenges in adhering to classical wide margin resections and limits the ability to be aggressive without unacceptable functional, physiological and aesthetic consequences (64,65).
The scope for definitive or adjuvant RT is limited as the RIS is located within previously irradiated tissue. As such, re-irradiation to a tumoricidal dose may lead to severe acute and late toxicities (such as osteoradionecrosis, soft tissue necrosis, radiation myelopathy and chronic non-healing ulcers) (62). Compromises in dose may mitigate these toxicities. However, this results in inadequate tumour control as sarcomas are inherently radio-resistant. The few studies reporting the use of re-irradiation, in conjunction with surgery, have predominantly been investigated in sites such as the thorax and extremities. Riad et al. reported improved local recurrence free interval with adjuvant RT (predominantly in extremities) of 7.7% vs. 34.5% (P=0.043) (66). About half of these patients, developed acute and/or late toxicity with re-RT. As such, re-RT must be used judiciously after having considered its risks and benefits. Factors to consider include previous irradiated volume, previous dose and fractionation regimen, dose received by critical organs and time elapsed since prior irradiation (30). Mitigation strategies for re-RT include using hyper fractionated RT regimes with smaller dose per fraction, highly conformal RT techniques such as intensity modulated proton therapy (IMPT), brachytherapy, using well-vascularised unirradiated tissue flaps during surgical resection and the use of concurrent chemotherapy allowing for a lower dose RT (67-71). These are elaborated further in the section below.
Despite aggressive resection, majority of the patients develop both local and distant relapses. Neuhaus et al. reported the outcome of 34 patients with RI soft tissue sarcoma who underwent curative resection. None of these patients received adjuvant RT, and 20% received neoadjuvant/adjuvant chemotherapy. In this cohort, the median survival was 54 months, with two-thirds of patients having local recurrence and 44% having distant relapse (20). In general, the use of chemotherapy, neo-adjuvant or adjuvant improves disease control. However, chemotherapy may not completely improve local control, as radiation induced fibrosis may impede chemotherapeutic agents from accumulating to adequate concentrations in the affected area (63). Unfortunately, the incremental survival benefit derived from chemotherapy is limited. It remains unclear if chemotherapy efficacy data can be extrapolated from de novo sarcomas. Experience from Italy, where 20 patients of radiation-induced osteosarcoma (involving the extremity) were treated with peri-operative multi-drug chemotherapy regimen (consisting of cisplatin, doxorubicin, methotrexate and ifosfamide) (72). Compared to patients with de novo osteosarcoma, the 5-year OS was inferior in the radiation induced osteosarcoma group (40 vs. 67%, P<0.01). In another series, 14 patients with RISHNN (of the calvarium or skull base) underwent resection and adjuvant chemotherapy (73). Five patients had R0 resection, four had R1 resection and the remaining had subtotal resection (R2). Only one patient remained disease-free after ~4 years of follow-up.
Prognosis
The prognosis of RISHNN is generally worse than patients with de novo sarcomas. These reasons include delays in diagnosis, inherently aggressive tumour behaviour, higher rates of local recurrence owing to challenges with complete surgical resection within previously irradiated fields, inability to deliver full-dose re-RT and limitations in chemotherapy choices due to prior radiation exposure.
For RIS in general, Gladdy et al. reported patients having a higher risk of death (HR 1.7; range, 1.1–2.4) compared to a matched cohort with de novo sarcoma (74). Similarly, data from the Norwegian Cancer registry reported significantly inferior survival of patients with RIS compared to de novo sarcoma (5 year survival 32 vs. 51%) (18). The French Sarcoma Group reported slightly more encouraging outcomes for RIS patients who managed to achieve R0 resection, with a 5-year survival rate of ~50% (75).
Looking only at patients with RISHNN, Coca-Pelaz performed a systematic review and reported the median survival to be ~13 months (22). Data from National Cancer Centre Singapore, compared the outcomes of patients with RISHNN (n=28) to de novo head and neck sarcoma (n=60) (12). In general, the survival of patients of RISHNN was worse. However, if patients were able to be treated with a curative intent, there was no significant survival difference between the two groups. As such, despite the poor prognosis, localised RIS should still be managed aggressively as they are still potentially curable with well-planned R0 surgery.
Mitigation strategies
As RISHNN is known to be an aggressive condition with a dismal prognosis, the premise of mitigation would be prevention through optimal patient and treatment selection.
Patient selection
Patient factors such as age at time of radiation exposure, volume of treatment and expected survival after radiation should be considered during decision-making. Young patients can be considered for curative treatment, avoiding RT where possible. Although there is no universally agreed age group of young patients, one can consider the remaining life-expectancy of the patient taking into account their underlying malignancy and co-morbidities. For early-stage tumors, organ preservation can still be achieved with limited surgery followed by close surveillance. An example of this would be a patient with Stage 1 glottic cancer undergoing trans-oral laser surgery. For advanced tumors, this may be achieved through more radical surgery with clear resection margins. Neoadjuvant chemotherapy can be considered for tumour down-staging prior to surgical resection.
Selection of radiation therapy techniques
This can be achieved through minimizing exposure of normal tissues and organs to radiation. This strategy is two-pronged. In terms of treatment volumes, elective nodal irradiation should be avoided if the risk of isolated nodal relapse is low (e.g., below 10–15% risk), especially in young patients. Secondly, compared to conventional photon therapy, modalities such as charged particle therapy (e.g., proton/ion beam) or brachytherapy are able to reduce unintended radiation exposure of normal tissue. The finite range of charged particle therapy, exhibited by the Bragg Peak effect, confers an advantage in avoiding radiation exposure distal to the target. However, the proximal areas are still invariably radiated, and therefore it is critical to note that the use of proton therapy does not eliminate the risk of RIS. In support of this, data from Xiang et al., using the National Cancer Database, does report a risk reduction of secondary malignancy with the use of proton beam therapy compared to IMRT (adjusted OR 0.31; 95% CI: 0.26–0.36, P<0.0001) (76). However, it is important to note that only 1.3% of this cohort were treated with proton beam therapy. This information has to be balanced with the fact that (I) the overall risk of secondary malignancies are generally low (~1.5 per 100 patient-years), (II) proton therapy is significantly more costly and (III) world-wide access and utility to proton therapy is currently limited.
Brachytherapy (as a monotherapy) has a limited role in head and neck cancers, being limited to early-stage oral cavity cancers (e.g., lip, mobile tongue) (77,78). As such, there is limited data to suggest if this can be used as a mitigating strategy. Extrapolating from uterine cancer where brachytherapy is commonly used, external beam therapy has a 44% (95% CI: 19–75%) higher risk of secondary malignancies compared to brachytherapy alone (79). However, in the context of RISHNN, this should only be considered as hypothesis-generating data.
Conclusions
Curative RT is a cornerstone in the management of head and neck cancers—resulting in many patients overcoming the initial cancer—but living with potential long-term risk of developing radiation-induced secondary malignancies. Amongst this, RISHNN is a particularly challenging condition to manage. Although there are no pathognomonic features of RISHNN, compared to de novo head and neck sarcomas, exposure to previous therapeutic radiation gives rise to this clinical diagnosis. It is imperative for oncologists to counsel patients (who have high probability of long-term survival) undergoing curative head and neck RT about this rare consequence. To date, there are no specific genetic studies suggestive of a causative mechanism. The preferred management for patients with localised RISHNN should be surgical resection, with or without reconstruction, with clear margins. However, we are cognizant that given the anatomical complexities with head and neck cancers, resection with clear margins may be challenging to achieve. In this case, the patient may be better served with a palliative treatment regime that maximizes quality of life.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dx.doi.org/10.21037/pcm-21-30
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/pcm-21-30). BV serves as an unpaid editorial board member of Precision Cancer Medicine from June 2020 to May 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pulte D, Brenner H. Changes in survival in head and neck cancers in the late 20th and early 21st century: a period analysis. Oncologist 2010;15:994-1001. [Crossref] [PubMed]
- Hall EJ. The inaugural Frank Ellis Lecture--latrogenic cancer: the impact of intensity-modulated radiotherapy. Clin Oncol (R Coll Radiol) 2006;18:277-82. [Crossref] [PubMed]
- Mark RJ, Bailet JW, Poen J, et al. Postirradiation sarcoma of the head and neck. Cancer 1993;72:887-93. [Crossref] [PubMed]
- Amendola BE, Amendola MA, McClatchey KD, et al. Radiation-associated sarcoma: a review of 23 patients with postradiation sarcoma over a 50-year period. Am J Clin Oncol 1989;12:411-5. [Crossref] [PubMed]
- Xi M, Liu MZ, Wang HX, et al. Radiation-induced sarcoma in patients with nasopharyngeal carcinoma: a single-institution study. Cancer 2010;116:5479-86. [Crossref] [PubMed]
- Bjerkehagen B, Smeland S, Walberg L, et al. Radiation-induced sarcoma: 25-year experience from the Norwegian Radium Hospital. Acta Oncol 2008;47:1475-82. [Crossref] [PubMed]
- Dineen SP, Roland CL, Feig R, et al. Radiation-Associated Undifferentiated Pleomorphic Sarcoma is Associated with Worse Clinical Outcomes than Sporadic Lesions. Ann Surg Oncol 2015;22:3913-20. [Crossref] [PubMed]
- CAHAN WG. WOODARD HQ. Sarcoma arising in irradiated bone; report of 11 cases. Cancer 1948;1:3-29. [Crossref] [PubMed]
- Murray EM, Werner D, Greeff EA, et al. Postradiation sarcomas: 20 cases and a literature review. Int J Radiat Oncol Biol Phys 1999;45:951-61. [Crossref] [PubMed]
- Debnam JM, Guha-Thakurta N, Mahfouz YM, et al. Radiation-associated head and neck sarcomas: spectrum of imaging findings. Oral Oncol 2012;48:155-61. [Crossref] [PubMed]
- Chan JY, Wong ST, Lau GI, et al. Postradiation sarcoma after radiotherapy for nasopharyngeal carcinoma. Laryngoscope 2012;122:2695-9. [Crossref] [PubMed]
- Yeang MS, Tay K, Ong WS, et al. Outcomes and prognostic factors of post-irradiation and de novo sarcomas of the head and neck: a histologically matched case-control study. Ann Surg Oncol 2013;20:3066-75. [Crossref] [PubMed]
- Yang Q, Mo Y, Zhao Q, et al. Radiation-induced sarcomas of the head and neck in post-radiation nasopharyngeal carcinoma. Radiol Med 2017;122:53-60. [Crossref] [PubMed]
- Wei Z, Xie Y, Xu J, et al. Radiation-induced sarcoma of head and neck: 50 years of experience at a single institution in an endemic area of nasopharyngeal carcinoma in China. Med Oncol 2012;29:670-6. [Crossref] [PubMed]
- Taghian A, de Vathaire F, Terrier P, et al. Long-term risk of sarcoma following radiation treatment for breast cancer. Int J Radiat Oncol Biol Phys 1991;21:361-7. [Crossref] [PubMed]
- Sheth GR, Cranmer LD, Smith BD, et al. Radiation-induced sarcoma of the breast: a systematic review. Oncologist 2012;17:405-18. [Crossref] [PubMed]
- Yap J, Chuba PJ, Thomas R, et al. Sarcoma as a second malignancy after treatment for breast cancer. Int J Radiat Oncol Biol Phys 2002;52:1231-7. [Crossref] [PubMed]
- Bjerkehagen B, Småstuen MC, Hall KS, et al. Incidence and mortality of second sarcomas - a population-based study. Eur J Cancer 2013;49:3292-302. [Crossref] [PubMed]
- Thijssens KM, van Ginkel RJ, Suurmeijer AJ, et al. Radiation-induced sarcoma: a challenge for the surgeon. Ann Surg Oncol 2005;12:237-45. [Crossref] [PubMed]
- Neuhaus SJ, Pinnock N, Giblin V, et al. Treatment and outcome of radiation-induced soft-tissue sarcomas at a specialist institution. Eur J Surg Oncol 2009;35:654-9. [Crossref] [PubMed]
- Mavragani IV, Nikitaki Z, Souli MP, et al. Complex DNA Damage: A Route to Radiation-Induced Genomic Instability and Carcinogenesis. Cancers (Basel) 2017;9:91. [Crossref] [PubMed]
- Coca-Pelaz A, Mäkitie AA, Strojan P, et al. Radiation-Induced Sarcomas of the Head and Neck: A Systematic Review. Adv Ther 2021;38:90-108. [Crossref] [PubMed]
- Makimoto Y, Yamamoto S, Takano H, et al. Imaging findings of radiation-induced sarcoma of the head and neck. Br J Radiol 2007;80:790-7. [Crossref] [PubMed]
- Sheppard DG, Libshitz HI. Post-radiation sarcomas: a review of the clinical and imaging features in 63 cases. Clin Radiol 2001;56:22-9. [Crossref] [PubMed]
- Ramsey RG, Zacharias CE. MR imaging of the spine after radiation therapy: easily recognizable effects. AJR Am J Roentgenol 1985;144:1131-5. [Crossref] [PubMed]
- NCCN. Bone cancer (version 2.2019). Available online: https://www.nccn.org/professionals/physician_gls/pdf/bone.pdf
- NCCN. Sarcoma. Available online: https://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf
- Edeiken B, deSantos LA. Percutaneous needle biopsy of the irradiated skeleton. Radiology 1983;146:653-5. [Crossref] [PubMed]
- Radiation-Induced Soft Tissue Sarcoma. Diagnosis, Treatment and Prognosis: Liddy Shriver Sarcoma Initiative. Available online: http://sarcomahelp.org/radiation-induced-sarcoma.html
- Thiagarajan A, Iyer NG. Radiation-induced sarcomas of the head and neck. World J Clin Oncol 2014;5:973-81. [Crossref] [PubMed]
- Zhu W, Hu F, Zhao T, et al. Clinical Characteristics of Radiation-Induced Sarcoma of the Head and Neck: Review of 15 Cases and 323 Cases in the Literature. J Oral Maxillofac Surg 2016;74:283-91. [Crossref] [PubMed]
- Inoue YZ, Frassica FJ, Sim FH, et al. Clinicopathologic features and treatment of postirradiation sarcoma of bone and soft tissue. J Surg Oncol 2000;75:42-50. [Crossref] [PubMed]
- Thariat J, Italiano A, Collin F, et al. Not all sarcomas developed in irradiated tissue are necessarily radiation-induced--spectrum of disease and treatment characteristics. Crit Rev Oncol Hematol 2012;83:393-406. [Crossref] [PubMed]
- Malone ER, Anderson N, Lewin JH, et al. Immune signature and molecular profiling of radiation-induced sarcoma (RIS). J Clin Oncol 2019;37:11040. [Crossref]
- Panse G, Mito JK, Ingram DR, et al. Radiation-associated sarcomas other than malignant peripheral nerve sheath tumours demonstrate loss of histone H3K27 trimethylation†. Histopathology 2021;78:321-6. [Crossref] [PubMed]
- Käcker C, Marx A, Mössinger K, et al. High frequency of MYC gene amplification is a common feature of radiation-induced sarcomas. Further results from EORTC STBSG TL 01/01. Genes Chromosomes Cancer 2013;52:93-8. [Crossref] [PubMed]
- Mito JK, Qian X, Jo VY, et al. MYC expression has limited utility in the distinction of undifferentiated radiation-associated sarcomas from sporadic sarcomas and sarcomatoid carcinoma. Histopathology 2020;77:667-72. [Crossref] [PubMed]
- Hadj-Hamou NS, Laé M, Almeida A, et al. A transcriptome signature of endothelial lymphatic cells coexists with the chronic oxidative stress signature in radiation-induced post-radiotherapy breast angiosarcomas. Carcinogenesis 2012;33:1399-405. [Crossref] [PubMed]
- Hadj-Hamou NS, Ugolin N, Ory C, et al. A transcriptome signature distinguished sporadic from postradiotherapy radiation-induced sarcomas. Carcinogenesis 2011;32:929-34. [Crossref] [PubMed]
- Berrington de Gonzalez A, Kutsenko A, Rajaraman P. Sarcoma risk after radiation exposure. Clin Sarcoma Res 2012;2:18. [Crossref] [PubMed]
- Henderson TO, Rajaraman P, Stovall M, et al. Risk factors associated with secondary sarcomas in childhood cancer survivors: a report from the childhood cancer survivor study. Int J Radiat Oncol Biol Phys 2012;84:224-30. [Crossref] [PubMed]
- Tucker MA, D'Angio GJ, Boice JD Jr, et al. Bone sarcomas linked to radiotherapy and chemotherapy in children. N Engl J Med 1987;317:588-93. [Crossref] [PubMed]
- Samartzis D, Nishi N, Cologne J, et al. Ionizing radiation exposure and the development of soft-tissue sarcomas in atomic-bomb survivors. J Bone Joint Surg Am 2013;95:222-9. [Crossref] [PubMed]
- Samartzis D, Nishi N, Hayashi M, et al. Exposure to ionizing radiation and development of bone sarcoma: new insights based on atomic-bomb survivors of Hiroshima and Nagasaki. J Bone Joint Surg Am 2011;93:1008-15. [Crossref] [PubMed]
- Kuttesch JF Jr, Wexler LH, Marcus RB, et al. Second malignancies after Ewing's sarcoma: radiation dose-dependency of secondary sarcomas. J Clin Oncol 1996;14:2818-25. [Crossref] [PubMed]
- Rubino C, Shamsaldin A, Lê MG, et al. Radiation dose and risk of soft tissue and bone sarcoma after breast cancer treatment. Breast Cancer Res Treat 2005;89:277-88. [Crossref] [PubMed]
- Kalra S, Grimer RJ, Spooner D, et al. Radiation-induced sarcomas of bone: factors that affect outcome. J Bone Joint Surg Br 2007;89:808-13. [Crossref] [PubMed]
- Wu LC, Kleinerman RA, Curtis RE, et al. Patterns of bone sarcomas as a second malignancy in relation to radiotherapy in adulthood and histologic type. Cancer Epidemiol Biomarkers Prev 2012;21:1993-9. [Crossref] [PubMed]
- Giannini L, Incandela F, Fiore M, et al. Radiation-Induced Sarcoma of the Head and Neck: A Review of the Literature. Front Oncol 2018;8:449. [Crossref] [PubMed]
- Filippi AR, Vanoni V, Meduri B, et al. Intensity Modulated Radiation Therapy and Second Cancer Risk in Adults. Int J Radiat Oncol Biol Phys 2018;100:17-20. [Crossref] [PubMed]
- van de Water TA, Lomax AJ, Bijl HP, et al. Potential benefits of scanned intensity-modulated proton therapy versus advanced photon therapy with regard to sparing of the salivary glands in oropharyngeal cancer. Int J Radiat Oncol Biol Phys 2011;79:1216-24. [Crossref] [PubMed]
- Stathakis S, Roland T, Papanikolaou N, et al. A prediction study on radiation-induced second malignancies for IMRT treatment delivery. Technol Cancer Res Treat 2009;8:141-8. [Crossref] [PubMed]
- Gupta T, Sinha S, Ghosh-Laskar S, et al. Intensity-modulated radiation therapy versus three-dimensional conformal radiotherapy in head and neck squamous cell carcinoma: long-term and mature outcomes of a prospective randomized trial. Radiat Oncol 2020;15:218. [Crossref] [PubMed]
- Schneider U, Lomax A, Pemler P, et al. The impact of IMRT and proton radiotherapy on secondary cancer incidence. Strahlenther Onkol 2006;182:647-52. [Crossref] [PubMed]
- Kry SF, Salehpour M, Followill DS, et al. The calculated risk of fatal secondary malignancies from intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys 2005;62:1195-203. [Crossref] [PubMed]
- Williams PO, Hounsell AR. X-ray leakage considerations for IMRT. Br J Radiol 2001;74:98-100. [Crossref] [PubMed]
- Ruben JD, Davis S, Evans C, et al. The effect of intensity-modulated radiotherapy on radiation-induced second malignancies. Int J Radiat Oncol Biol Phys 2008;70:1530-6. [Crossref] [PubMed]
- Ding GX, Coffey CW. Radiation dose from kilovoltage cone beam computed tomography in an image-guided radiotherapy procedure. Int J Radiat Oncol Biol Phys 2009;73:610-7. [Crossref] [PubMed]
- Kim DW, Chung WK, Yoon M. Imaging doses and secondary cancer risk from kilovoltage cone-beam CT in radiation therapy. Health Phys 2013;104:499-503. [Crossref] [PubMed]
- Patel J, Baptiste BA, Kim E, et al. DNA damage and mitochondria in cancer and aging. Carcinogenesis 2020;41:1625-34. [Crossref] [PubMed]
- Hisada M, Garber JE, Fung CY, et al. Multiple primary cancers in families with Li-Fraumeni syndrome. J Natl Cancer Inst 1998;90:606-11. [Crossref] [PubMed]
- Spałek MJ, Czarnecka AM, Rutkowski P. The Management of Radiation-Induced Sarcomas: A Cohort Analysis from a Sarcoma Tertiary Center. J Clin Med 2021;10:694. [Crossref] [PubMed]
- Lagrange JL, Ramaioli A, Chateau MC, et al. Sarcoma after radiation therapy: retrospective multiinstitutional study of 80 histologically confirmed cases. Radiation Therapist and Pathologist Groups of the Fédération Nationale des Centres de Lutte Contre le Cancer. Radiology 2000;216:197-205. [Crossref] [PubMed]
- Cha C, Antonescu CR, Quan ML, et al. Long-term results with resection of radiation-induced soft tissue sarcomas. Ann Surg 2004;239:903-9; discussion 909-10. [Crossref] [PubMed]
- Rosko AJ, Birkeland AC, Chinn SB, et al. Survival and Margin Status in Head and Neck Radiation-Induced Sarcomas and De Novo Sarcomas. Otolaryngol Head Neck Surg 2017;157:252-9. [Crossref] [PubMed]
- Riad S, Biau D, Holt GE, et al. The clinical and functional outcome for patients with radiation-induced soft tissue sarcoma. Cancer 2012;118:2682-92. [Crossref] [PubMed]
- de Jong MA, Oldenborg S, Bing Oei S, et al. Reirradiation and hyperthermia for radiation-associated sarcoma. Cancer 2012;118:180-7. [Crossref] [PubMed]
- Stuschke M, Kaiser A, Abu-Jawad J, et al. Re-irradiation of recurrent head and neck carcinomas: comparison of robust intensity modulated proton therapy treatment plans with helical tomotherapy. Radiat Oncol 2013;8:93. [Crossref] [PubMed]
- Naghavi AO, Gonzalez RJ, Scott JG, et al. Staged reconstruction brachytherapy has lower overall cost in recurrent soft-tissue sarcoma. J Contemp Brachytherapy 2017;9:20-9. [Crossref] [PubMed]
- Götzl R, Sterzinger S, Arkudas A, et al. The Role of Plastic Reconstructive Surgery in Surgical Therapy of Soft Tissue Sarcomas. Cancers (Basel) 2020;12:3534. [Crossref] [PubMed]
- Garg S, Kilburn JM, Lucas JT Jr, et al. Reirradiation for second primary or recurrent cancers of the head and neck: Dosimetric and outcome analysis. Head Neck 2016;38:E961-9. [Crossref] [PubMed]
- Bacci G, Forni C, Longhi A, et al. Local recurrence and local control of non-metastatic osteosarcoma of the extremities: a 27-year experience in a single institution. J Surg Oncol 2007;96:118-23. [Crossref] [PubMed]
- Patel AJ, Rao VY, Fox BD, et al. Radiation-induced osteosarcomas of the calvarium and skull base. Cancer 2011;117:2120-6. [Crossref] [PubMed]
- Gladdy RA, Qin LX, Moraco N, et al. Do radiation-associated soft tissue sarcomas have the same prognosis as sporadic soft tissue sarcomas? J Clin Oncol 2010;28:2064-9. [Crossref] [PubMed]
- Italiano A, Bringer S, Blay JY, et al. Patterns of Care and Outcome Radiation-Induced Soft Tissue Sarcomas. Int J Radiat Oncol Biol Phys 2019;103:449-52. [Crossref] [PubMed]
- Xiang M, Chang DT, Pollom EL. Second cancer risk after primary cancer treatment with three-dimensional conformal, intensity-modulated, or proton beam radiation therapy. Cancer 2020;126:3560-8. [Crossref] [PubMed]
- Kovács G, Martinez-Monge R, Budrukkar A, et al. GEC-ESTRO ACROP recommendations for head & neck brachytherapy in squamous cell carcinomas: 1st update - Improvement by cross sectional imaging based treatment planning and stepping source technology. Radiother Oncol 2017;122:248-54. [Crossref] [PubMed]
- Bhalavat R, Budrukkar A, Laskar SG, et al. Brachytherapy in head and neck malignancies: Indian Brachytherapy Society (IBS) recommendations and guidelines. J Contemp Brachytherapy 2020;12:501-11. [Crossref] [PubMed]
- Lönn S, Gilbert ES, Ron E, et al. Comparison of second cancer risks from brachytherapy and external beam therapy after uterine corpus cancer. Cancer Epidemiol Biomarkers Prev 2010;19:464-74. [Crossref] [PubMed]
Cite this article as: Chan TY, Alagappan V, Vellayappan B. Wielding a double-edged sword in head and neck cancers—diagnosis, risk factors and mitigation strategies of radiation-induced head and neck sarcomas: a narrative review. Precis Cancer Med 2021;4:36.