
Metabolic reprogramming and cancer precision medicine: a narrative review
Introduction
Precision medicine in cancer aims to improve outcome of cancer patients by using customized strategy to prevent, diagnose and treat cancer according to tumor molecular features. As the metabolic reprogramming empowers cancer cells flexibility to adapt stress and microenvironment, metabolic reprogramming is critical for tumorigenesis, cancer progression, tumor responses upon treatment, and therapy resistance. Therefore, targeting cancer metabolism can provide effective approaches for cancer precision management. Increasing research in cancer metabolism investigate how reprogrammed metabolism works and drives rapid cell proliferation, or which reprogrammed activities are relevant to resistance of treatment. Deep understanding of these molecular insights about cancer metabolism is the foundation for design of effective cancer management and therapeutic strategies for different cancer types.
Tumor metabolism is a complex progress dynamically affected by intrinsic and extrinsic factors, which results in high metabolic heterogeneity of cancer. Cancer cell intrinsic effects of oncogene include perturbed signaling, genetic mutations, gene expressions and so on. While extrinsic factors depend on the tumor microenvironment (TME) and systematical metabolism of the patient (1). There are key characteristics of cancer cells, for example, enhanced aerobic glycolysis, mutations in the tricarboxylic acid (TCA) cycle metabolic enzymes, and dependence on lipid and glutamine metabolism. Moreover, cancer cells switch metabolic pathways to avoid cell death upon anti-cancer drugs. Therefore, targeting metabolic plasticity and flexibility is expected to decrease anti-cancer drug resistance (2). Collectively, cancer metabolic heterogeneity and flexibility bring in big challenges for cancer treatment. And targeting cancer metabolic reprogramming is critical for cancer precise treatment and management.
We present the following article in accordance with the Narrative Review reporting checklist (available at: https://dx.doi.org/10.21037/pcm-21-27).
Methods
Here, this review aims to summarize major characteristics and updates of cancer metabolism, focusing on how to target cancer metabolic reprogramming for cancer precision medicine (Figure 1). Specifically, we discussed the key updates of cancer metabolic reprogramming, metabolic targets for cancer therapy (Figure 2), precision nutrition and cancer treatment (Figure 3), and metabolic imaging for cancer management.
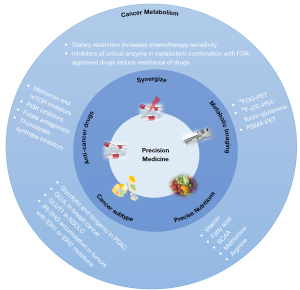
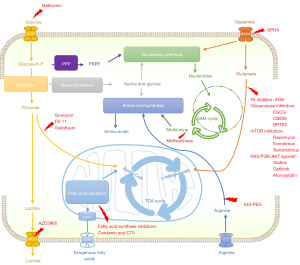
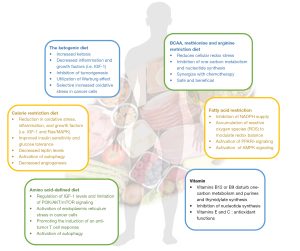
Cancer metabolic reprogramming
Warburg effect and cancer diagnosis
Hyperactive glycolysis is the common and important feature of many cancers. Normal cells oxidize glucose to CO2 through mitochondrial oxidative phosphorylation to efficiently produce adenosine triphosphate (ATP) when oxygen is enough, and use glucose to produce lactic acid through fermentation (3) under hypoxia condition, which is a fast metabolic pathway but low-efficient in producing ATP. Nevertheless, cancer cells prefer to a glycolytic form for fulfill their bioenergetic and biosynthetic needs of rapid energy generation even if oxygen is available, which is known as Warburg Effect (4). In addition to ATP generation, multiple intermediate products of glucose metabolism feed into biosynthetic pathways to produce lipids, nucleotides and synthesis of macromolecules to support tumor growth (5). Moreover, emerging evidence shows that lactic acid is a regulator participating in tumor progression (6). Abundant lactic acid generated from glycolysis not only fuels tumor (7,8), but also creates an immune suppressive microenvironment to promote tumor progression. Lactic acid limits retinoic acid-inducible gene I (RIG-I)-like receptor (RLR)-mediated type I interferon (IFN) production to suppress innate immunity (9) and contributes to the intra/extracellular pH within TME to polarize tumor associated macrophages (10). The acidic extracellular pH can also promote the secretion of lysosomal enzymes, and induces the expression of pro-metastatic factors via intracellular signaling (6).The Warburg effect phenotype is commonly observed in many aggressive cancers (11,12). Base on the high glucose uptake and glycolysis of cancer cells, 18fluoro-2-deoxy-d-glucose (18F-FDG) positron emission tomography (PET) scan has been widely used in clinic as a diagnostic marker of tumor detection, stage, and prognostic evaluation (13). However, the limitation of 18F-FDG-PET in detecting prostate cancer and brain tumor, demonstrates that metabolic reprogramming other than glycolysis exists in cancers.
Mitochondrial metabolism and updates of Warburg effect
As an update of Warburg Effect that cancer cells prefer glycolysis while decrease mitochondrial oxidative phosphorylation, mitochondrial metabolism is found to be hyperactive and necessary for tumor growth in recent years (14). Mitochondria are well known as the principal organelles to produce ATP for the energy needs of cells. Carbon sources, such as pyruvate, glutamine, and fatty acids, generate NADH and FADH2 through the TCA cycle in mitochondrial matrix and transfer their electrons to the electron transport chain (ETC) which embedded in the inner mitochondrial membrane (15). Therefore, mitochondria are essential in biosynthetic and bioenergetic signaling and play a critical role in cell differentiation, proliferation, and death (16). Warburg effect firstly described mitochondria in malignant cancer cells is structurally and functionally different from normal cells (17), which promotes the dependence on glycolysis in cancer cells. However, rising evidence show a normal function of mitochondria in cancer. Recent studies reveal that mitochondrial metabolism supports tumor anabolism by providing key metabolites for macromolecule synthesis and generating oncometabolites to maintain the cancer phenotype. Multiple substrates feed into the biosynthetic pathways in mitochondrial, providing cancer cells with metabolic flexibility to support tumor growth under various conditions (14). And mitochondrial biogenesis and glycolysis in the p53 knockout mouse were significant upregulation at the same time. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function for survival (18). Moreover, targeting mitochondrial ETC can synergy the anti-cancer efficacy of immuno-, antiangiogenic, or oncogene targeted therapies. Mechanistically, tumor growth requires the ETC to oxidize ubiquinol, which is an essential activity of mitochondrial complex III that allows complex I, II, and dihydroorotate dehydrogenase (DHODH) to function. Therefore, ETC is essential to drive the oxidative TCA cycle and DHODH activity in cancer (19).
Targeting mitochondrial reprogramming offers important therapeutic targets for cancer patients. For instance, increased oxidative phosphorylation drives resistance to BCL-2 inhibition, Venetoclax, a FDA-approved BCL-2 inhibitor for chronic lymphocytic leukemia (CLL) (20) and acute myeloid leukemia (AML) (21). And inhibitors of the ETC complex III (antimycin A) and complex V (oligomycin), can promote Venetoclax sensitivity in CLL patient samples (22), supporting implementation of combinatorial therapy with metabolic modulators to address Venetoclax resistance (22). Moreover, targeting multiple tyrosine kinases in mitochondrial ETC complexes, Sorafenib induced intra-tumoral hypoxia and reactive oxygen species (ROS) production in sorafenib-resistant cells, by upregulated UBQLN1 induced peroxisome proliferator-activated receptor γ coactivator 1β (PGC1β) degradation (23). Furthermore, targeting complex I of ETC, IACS-010759 significantly inhibits tumor growth in ibrutinib-resistant patient-derived cancer models (24). These studies provide a clinically viable approach of subverting therapeutic resistance by targeting cancer mitochondrial oxidative phosphorylation.
Oxidative stress in cancer
Tumor cells continually accommodate to oxidative stress by NADPH production in different ways during tumorigenesis, such as stimulated AMP-activated protein kinase (AMPK), the pentose phosphate pathway (PPP), and reductive glutamine and folate metabolism (25). Notably, ROS usually work in distinctive ways in tumor initiation, progression, and metastasis (25). On one hand, increased myeloid cell-derived ROS and TNF-α mediated signaling can lead to epithelial mutagenesis and carcinogenesis (26). ROS can affect GAPDH, PKM2, and G6PD activities by coordination with Cys residues oxidation in the ETC within complexes I, III, and IV, resulting in cancer metabolic adaptation (27). On the other hand, oxidative stress modulates gene expression of downstream targets involved in DNA repair, cell proliferation and antioxidants (28). Oxidative stress limits distant metastasis by melanoma cells in vivo (29). Melanoma cells in the blood and visceral organs experienced higher oxidative stress than subcutaneous tumors. Successfully metastasizing melanomas underwent reversible metabolic changes during metastasis that increased their capacity to withstand oxidative stress, including increased dependence on NADPH-generating enzymes in the folate pathway. Antioxidants thereby promoted distant metastasis in mice (29). Moreover, high-OXPHOS tumor exhibit chemosensitivity through increased oxidative stress and PML (30). High-grade serous ovarian cancers (HGSOCs) show that chronic oxidative stress promotes aggregation of PML-nuclear bodies and activation of the transcriptional co-activator PGC-1α which increases synthesis of ETC complexes in high-OXPHOS tumors. OXPHOS metabolic heterogeneity in high-OXPHOS tumors, promotes aggregation of PML-nuclear bodies that activate PGC-1α, ETC synthesis, and mitochondrial respiration (30). Therefore, antioxidants need to be used with caution in cancer, depending on the oxidative stress status of cancer.
Many transcription factors (TFs), including nuclear factor erythroid 2-related factor 2 (Nrf2), hypoxia inducible factor 1 α (HIF-1α), activator protein 1 (AP-1), heat shock factor 1 (HSF1), nuclear factor κB (NF-κB) and tumor protein p53 can be activated by ROS and regulate the redox status as feedback (31). Nrf2, a vital regulator in cell signaling transduction, is a highlighted stress-responsive TF and was considered as an antioxidative TF. Nrf2 also regulates metabolic flexibility of cancer cells upon different ROS levels. Nrf2 regulated antioxidant defense is required by activation of the AMPK-HIF-1α signaling in high ROS condition, while Nrf2 was not required in low ROS condition (32). In addition, HIF-1α increases expression of GSH-based antioxidant genes under hypoxic conditions. And chemotherapy including paclitaxel, gemcitabine and carboplatin triggers HIF-1–dependent glutathione synthesis, which induces the enrichment of breast cancer stem cells (33). Collectively, HIF1a-Nrf2 signaling plays critical role in regulating cancer cell adaptation to oxidative stress.
Glutaminolysis and cancer
As the most abundant circulating amino acid (AA) in blood, glutamine plays vital roles in cancer metabolism including synthesis of metabolites that maintain mitochondrial metabolism, balances the redox homeostasis and activation of cell signaling (34). Glutamine is converted to glutamate by glutaminase (GLS). Glutamate is deaminated to α-ketoglutarate (α-KG) by catalyzing of glutamate dehydrogenase (GLUD) or transaminases. α-KG further enter into TCA cycle to produce energy (35). In cancer, glutamine can not only fulfill the TCA cycle to provide energy and intermediate metabolites for fast proliferating cancer cells, but also serve as a signaling molecule to stimulate the mechanistic target of rapamycin complex 1 (mTORC1) pathway and promote cell growth (36). For instance, cancer cells with mutant KRAS can promote glutaminolysis and activate Nrf2 antioxidant signaling to increase tumor progression (37). Accordingly, mitochondria derived ROS are essential for KRAS-induced cell proliferation and tumorigenesis by regulation of the ERK1/2 MAPK pathway in mouse model of lung cancer (38). A recent study suggested that inhibition of glutaminolysis can increase gemcitabine sensitivity in gemcitabine-resistant pancreatic cancer cells (39). And a shift in glutamine nitrogen metabolism contributes to the malignant progression of cancer (40). Therefore, many inhibitors of key enzymes in glycolysis and glutaminolysis pathways has been proven beneficial to in suppression tumor growth in clinical trials (41). Importantly, the SLC1A5 (also known as ASCT2) variant has recently been identified as a mitochondrial glutamine transporter, with a specific mitochondrial targeting sequence induced by HIF-2α. The SLC1A5 variant mediates glutaminolysis and redox regulation in cancer cells. In consistent, SLC1A5 variant knockdown strongly suppressed cancer cell growth in pancreatic cancer (42). Collectively, targeting glutamine metabolism and mitochondrial glutamine transporters may become a new therapeutic strategy for controlling tumor growth.
Branched-chain amino acid (BCAA)
BCAA includes three essential AAs, leucine, isoleucine, and valine, which are not synthesized in humans and can only get from diet. BCAA play vital roles in protein synthesis, nutrition metabolism, gut and immune in humans (43). Notably, BCAA metabolism plays critical roles in cancers with hyperactivating RAS signaling. Elevation of circulating BCAA is an early event in human pancreatic adenocarcinoma development (44), majority of which harboring KRAS mutations. Further research revealed that BCAA transaminase 2 (BCAT2)-mediated BCAA catabolism promotes KRAS-driven pancreatic ductal adenocarcinoma (PDAC) development (45). KRAS can inhibit BCAT2 ubiquitylation and degradation to stabilize BCAT2, through mediation of TRIM21 E3 ligase by suppressing SYK induced Y228 phosphorylation. In consistent, BCAT2 is significantly upregulated in human PDAC samples and enhances BCAA uptake in PDAC cells to promote cancer cell growth and survival (45). In addition, another BCAA metabolic enzyme, BCAT1, have emerged as useful prognostic cancer markers. BCAT1 expression commonly correlates with more aggressive cancer growth and progression (46). BCAAs also support cancer growth as a fuel in melanoma, nasopharyngeal carcinoma, and breast cancer. Therefore, targeting BCAT2 or lowering dietary BCAA therefore have promising anti-cancer effect, especially for KRAS-driven cancers like PDAC (45).
One-carbon metabolism
One-carbon (1C) metabolism comprises a series of interlinking metabolic pathways that include the methionine and folate cycles that are central to cellular function, providing 1C units (methyl groups) for the synthesis of DNA, polyamines, AAs, creatine, and phospholipids (47). One-carbon metabolism can also produce glutathione to keep the redox homeostasis in the TME and provides substrates for the methylation reaction (48).
Agents targeting folate metabolism have been widely used in cancer chemotherapy, including two major drug groups: folate antagonists (e.g., methotrexate) and thymidylate synthase inhibitors (e.g., 5-fluorouracil). But in view of the important function of folate to normal cells, the administration of these drugs in cancer chemotherapy can induce a state of acute folate depletion with sometimes life-threatening toxic sequelae (49). Mitochondrial serine catabolism is considered the sole contributor of folate-mediated 1C units in proliferating cancer cells. A recent study report that under physiological folate levels in cell, cytosolic serine-hydroxymethyltransferase (SHMT1) is the predominant source of 1C units in a variety of cancers, while mitochondrial 1C flux is overly repressed (50). Moreover, tumor-specific reliance on cytosolic 1C flux is associated with poor capacity to retain intracellular folates, which is determined by the expression of SLC19A1. This study revealed major diversity in cancer cell utilization of the cytosolic versus mitochondrial folate cycle across tumors and SLC19A1 expression as a marker for increased reliance on SHMT1 (50), providing a possible way to optimize folate drugs for future precise use in cancer.
Methionine is a critical component of one-carbon metabolic network and the primary source for S-adenosyl-methionine (SAM), which is a methyl-donor for histone and DNA methylation. Notably, targeting methionine metabolism influences cancer therapy response. Dietary methionine restriction sensitizes PDX models of colorectal cancer to chemotherapy with 5-FU, and sensitizes mouse models of RAS driven autochthonous sarcoma to radiation (51). Moreover, pharmaceutical inhibition of MAT2A, key enzyme of methionine metabolism, blocks the growth of MTAP-deleted cancer cells by reducing PRMT5-dependent mRNA splicing and inducing DNA damage (52). But not all cancer cells are sensitive to methionine restriction or inhibition of methionine metabolism. MAT2A inhibitors that substantially reduce levels of S-adenosylmethionine (SAM), AGI-24152 and AG-270 significantly reduce proliferation of cancer cells and tumors that lack MTAP (52). And hepatocyte nuclear factor 4α (HNF4α) dictates the sensitivity of liver cancer to methionine restriction. Hepatic sulfur amino acid (SAA) metabolism is under transcriptional control of HNF4α. Knocking down HNF4α or SAA enzymes in HNF4α-positive epithelial liver cancer lines impairs SAA metabolism, increases resistance to methionine restriction or sorafenib, promotes epithelial-mesenchymal transition, and induces cell migration (53).
Serine is an important one-carbon donor to the folate cycle and many cancer cells highly rely on serine for nucleotide synthesis, methylation reactions and the generation of NADPH for antioxidant defence (54). MYCN overexpression in neuroblastoma cells activates the serine-glycine-one-carbon biosynthetic pathway and rely on this way for supplying glucose-derived carbon. Blocking the signaling in MYCN-amplified cell lines with small molecule inhibitors induces metabolic stress and autophagy, which may be exploited as a selective target (55). Moreover, 3-phosphoglycerate dehydrogenase (PHGDH) is recognized as the rate-limiting step during glucose-derived serine synthesis and plays a critical role in brain metastasis of multiple cancer types. Increased serine synthesis promotes nucleotide production and growth in cancer cells. Therefore, PHGDH inhibitors are potential drugs to treat brain metastasis, which have achieved good efficacy in preclinical models (56). Lastly, MDM2 overexpression mediates serine metabolism to sustain nucleotide synthesis and tumor growth in p53 wild-type liposarcomas. Targeting MDM2 functions in serine metabolism shows a potential therapeutic strategy for liposarcomas (57). All together, altered one-carbon metabolism is essential for cancer cell growth, which provides potential therapeutic targets for cancer treatment.
Immune metabolism and cancer therapy
Metabolism of immune cells can not only influence their differentiation and function, but also regulate cancer progression and cancer therapy response. Almost every immune cell type actively participates in regulation of cancer progression (58-60). On one hand, there is complex tumor-immune crosstalk that can modulate metabolic conditions in the TME (61). For example, the competitive environment of the TME promote TILs to enter an exhausted state. Exhausted CD8+ T (Tex) cells weaken effector function and metabolic dysregulation are up-regulated during chronic infection or cancer (62). Besides, glutamine blockade can promote both increased CD8+ TIL effector function and tumor suppression. Therefore, glutamine antagonism is exploited as a “metabolic checkpoint” to enhances antitumor immune response (63). On the other hand, immune metabolism and improve the efficacy of target immune checkpoint therapy, which has achieved striking clinical breakthrough in cancer immunotherapy in recent years (64-66). NAD+ replenishment increase the sensitivity of anti-PD-L1 antibody in immunotherapy-resistant tumors (67). Similarly, blocking retinoic acid production can synergize with anti-PD-1 therapy (68). Taken together, immune metabolism is tightly associated with cancer progression and cancer therapy response. Immune checkpoint blockade combination with regulation of metabolic modulators could be effective therapeutic strategy to increase immunotherapy.
Precision nutrition for cancer therapy
Tumor growth and proliferation rely on the nutrients from environment. Therefore, dietary interventions may provide novel strategy to influence tumor progression (Figure 3).
Calorie restriction (CR)
CR is a robust environmental intervention known to increase healthy life, prolong lifespan and decrease cancer incidence in mammalians (69). CR inhibits cancer through multiple molecular signaling pathway, mainly including hyperactivation of sirtuins, suppressing LKB1/AMPK and IGF1/PI3K/AKT/mTOR signaling transduction (69). Short-term fasting can also improve anticancer chemotherapy via T cells and autophagy (70). But fasting is difficult for patients, requiring pharmacological agents mimicking caloric restriction. While role of sirtuins vary among different cancer types, metformin and mTOR inhibitors have demonstrated the anti-cancer effect by suppressing the IGF1/PI3K/AKT/mTOR pathway. Caloric restriction mimetics (CRMs) also mimic the biochemical effects of nutrient deprivation by reducing lysine acetylation of cellular proteins, thus triggering autophagy. Short-term fasting or treatment with several chemically unrelated autophagy-inducing CRMs, including hydroxycitrate and spermidine, improved the inhibition of tumor growth by chemotherapy in vivo. Notably, this effect was only observed for autophagy-competent tumors, depended on the presence of T lymphocytes, and was accompanied by the depletion of regulatory T cells from the tumor bed (70). In addition, cancer cells with mutations in the PI3K signaling pathway are resistant to the growth inhibitory effects of caloric restriction, and genetically engineering an activating PI3K pathway mutation into cancer cells confer resistance to caloric restriction (71,72).
Fasting-mimicking diet (FMD)
FMD is a straightforward approach in various dietary interventions for cancer therapy, including acute intermittent fasting or short-term fasting. FMD can widely alter growth factors and metabolite levels to improve anti-tumor effect of cancer therapies to keep environments that can reduce the capability of cancer cells to adapt and survive. For example, FMD can increase the anti-tumor effect of tamoxifen and fulvestrant in HR+/HER2− breast cancer in vitro and in vivo (73). FMD can also selectivity reverses vitamin C-induced heme-oxygenase-1 and ferritin activation in KRAS-mutant cancer cells. And the combination of FMD and vitamin C represents a promising therapeutic strategy with low toxicity in KRAS mutated tumors (74,75). Moreover, fasting or FMD can increase resistance to chemotherapy in normal tissue but not cancer cells. In other words, FMD prevent damage and potentially side effects to normal tissues during clinical treatments (76). Therefore, FMD can synergize with cytotoxic chemotherapy or other antitumor therapies to protect normal tissues from chemotherapy-induced toxicity (77). However, a clinical trial showed the overall no beneficial effect of FMD during chemotherapy in breast cancer patients (77). More research is needed to evaluate the effect of FMD during cancer treatment in vivo and to find FMD-based therapeutic combinations.
The ketogenic diet (KD)
The KD, a low-carbohydrate with adequate calories diet, restricts both carbohydrates and protein intake to decrease blood glucose and reduce serum levels of insulin and insulin-like growth factor (IGF)-1, leading to change of glycolysis in tumor and decrease of tumor growth (76,78). Fatty acids from KD can be converted to β-hydroxybutyrate, acetoacetate and other ketones in the liver by β-oxidation. The ketone bodies then circulate in the bloodstream and be transported to acetyl-CoA to participate in the TCA cycle (79). Therefore, KD can provide enough energy for life activities while reduce blood glucose and insulin signaling. As a result, KD show great importance in affecting the efficacy of PI3K inhibitors. PI3K inhibitors suppress glucose uptake, resulting in elevated blood glucose and pancreatic insulin release after drug treatment. The increased levels of insulin in blood reactivates PI3K signaling and increases glucose uptake in the tumor, limits the efficacy of PI3K inhibitors on tumor growth by inhibition of the insulin-PI3K-AKT-mTOR signaling pathways (71,80). Additionally, tumor genetic background can also regulate tumor response to the KD, for example, KD enhances the growth of BRAFV600E melanoma (81,82).
Amino acid-defined diet
AAs play a critical role in tumor survival and growth, including providing carbon sources for the TCA, nitrogen sources for base synthesis, and regulation of redox balance. Targeting amino acid metabolism by depleting circulating blood AAs in dietary has become one of the focuses of modern tumor therapy. For instance, dietary serine and glycine starvation was confirmed to suppress tumor growth in intestinal cancer and lymphoma mouse model (83). Whereas, reducing essential AAs also can disturb the living state of normal tissues, this potential strategy is more suitable for auxotrophic tumors, which relying on the external supply of AAs without the synthesizing of one specific nonessential AA (84).
BCAA includes three essential amino acids, leucine, isoleucine, and valine. BCAA metabolism is mainly carried out in muscle tissue, it is an important nutrient source and catabolized as substrates by highly reversible enzymes (43). Dietary restriction of BCAA inhibits the resistance of insulin in obese mice treated with high fat/high sugar (85), and limit the development and progression of some tumors (46), like liver cancer development (86) and KRAS-driven PDAC (45).
Methionine deprivation can reduce cellular redox stress (87), inhibit one-carbon metabolism and nucleotide synthesis (51). Dietary methionine restriction to enhance anti-tumor effect was demonstrated in various cancer cells and mouse models (88). Methionine-restricted diet also can synergize with various chemotherapies and radiotherapy (89,90). Moreover, synergies between methionine restriction and 5-fluorouracil, the inhibitor of one-carbon metabolism is demonstrated in many studies (91,92). Notably, methionine restriction is not beneficial for all cancer. And tumors that lack MTAP (52) and HNF4α (53) dictates the sensitivity of liver cancer to methionine restriction. In these studies, methionine restriction is a safe and beneficial way to fight with cancer.
Arginine is a nonessential AA required for protein synthesis, participating in nitric oxide production and the synthesis of nucleotides, polyamine, creatine, proline, glutamate, or urea. Arginine limitation is advantageous to tumor cells, allowing cytosolic aspartate used for pyrimidine production to take part in RNA and DNA synthesis (93). For example, arginine deficiency therapy is worked well in small-cell lung cancers driven by MYC (94). Arginine restriction by ADI-PEG20 treatment has been proved to synergize with chemotherapy in sarcoma (95). Therefore, tumor cells with metabolic defect become dependent on an exogenous arginine supply, a vulnerability that can be exploited for therapy.
Fatty acid restriction
Aberrant lipid metabolism occurs in many human cancers. Fatty acid oxidation (FAO) has been reported to be preferred and activated in breast cancer (96,97), such as in MYC-overexpressing triple-negative breast cancer (98), and CPT1C overexpression cancers (99). NADPH is a key metabolite in redox balance and FAO can produce NADPH to keep cancer cells survival under metabolic stress. Inhibition of FAO can accumulate ROS and reduce tumor growth in glioma cells (100). And tumor cells can keep their rapid proliferation state with NADPH supply through AMPK signaling (101). Therefore, high-fat diet (HFD) can increase the risks of adenomas and carcinomas in the intestine. And HFD increase aberrant proliferation in xenograft by activation of PPARδ (102). Consistently, a low-fat diet (LFD) is reported can prolong the survival of obese leukemia-bearing mice by inhibition of FAO in mouse model (103). Restriction of nutritional fatty acids may be a potential therapy to starve some cancers (104).
Vitamin
Vitamin deficiencies have been linked to the development of certain cancers (105). For example, vitamin B12 or B9 restriction can disturb one-carbon metabolism and purines and thymidylate synthesis (88). Limitation of vitamins B12 or B9 can impede cancer progression (106). Vitamins B9 also named folate is an essential vitamin for nucleotide synthesis and is essential for cell proliferation, especially for rapidly proliferating intestinal epithelial cells, hematopoietic cells and tumor cells. Combination with dietary uridine supplementation, folate restriction decrease the growth of intestinal tumors in Apcmin/+ mice (107). In addition, vitamins E and C as dietary supplements can suppress cancer development due to their antioxidant functions (108,109). Intravenous vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH (108), providing a mechanistic rationale for exploring the therapeutic use of vitamin C for CRCs with KRAS or BRAF mutations (108). High-dose vitamin C also enhances cancer immunotherapy (110). Vitamin C not only enhances the cytotoxic activity of adoptively transferred CD8 T cells but also cooperates with immune checkpoint therapy in several cancer types (110).
Taken together, diet-mediated changes in whole-body metabolism and systemic nutrient availability can influence the microenvironment and macroenvironment of cancer cells. Precise dietary interventions provide new approaches to help cancer patients fight against cancer.
Metabolic imaging and precise cancer management
Glucose metabolism imaging and cancer management
Hyperactivated glycolysis exist in many solid tumors. Accordingly, 18fluorodeoxyglucose (18F-FDG) PET/CT to detect tumor glucose uptake and glycolysis status has been widely used in cancer management. In addition to sensitive detection of primary tumor lesions, the powerful function of 18F-FDG PET/CT in detecting whole-body lymphoid nodes and distant metastasis makes it widely used in cancer stage evaluation, therapy response monitoring, prognosis prediction, and radiotherapy planning for multiple cancer types. Notably, early changes of 18F-FDG-PET maximum standardized uptake value (Δ18F-FDG-PET SUVmax) upon cancer treatment can not only serve as nonspecific responses biomarker to chemotherapy, but also as specific responses biomarker to targeted therapy that regulates glucose metabolism like PI3K inhibitors (111). Therefore, 18F-FDG PET/CT greatly improves clinical decision-making for physicians, as well as diagnosis and treatment for cancer patients.
Glutamine imaging and brain tumor management
Despite that 18F-FDG PET imaging has valuable implications in most solid tumor management, 18F-FDG is ineffective in evaluating gliomas because of high background uptake in the brain (112). Glutamine addiction, known as a high rate of glutamine consumption normally exceeding cellular biosynthetic and energetic needs, significantly exists in brain tumor cells (113), and is dispensable for glioma cell growth (114). Importantly, PET imaging in vivo with the glutamine analogue 4-18F-(2S,4R)-fluoroglutamine (18F-FGln) shows high uptake in gliomas but low background brain uptake, facilitating clear tumor delineation (112). Chemo/radiation therapy reduced 18F-FGln-tumor avidity, corresponding with decreased tumor burden. 18F-FGln also showed high tumor/background ratios with minimal uptake in the surrounding brain in human glioma patients with progressive disease (112). A clinical trial further assessed the clinical safety, pharmacokinetics, and tumor imaging characteristics of 18F-FGln (115). FGln PET depicted tumors of different cancer types (breast, pancreas, renal, neuroendocrine, lung, colon, lymphoma, bile duct, or glioma) in 17 of the 25 patients, predominantly clinically aggressive tumors with genetic mutations implicated in abnormal glutamine metabolism (115). These studies demonstrated that 18F-FGln is avidly taken up by gliomas, and could serve as a valuable tool in the clinical management of gliomas.
Metabolic imaging for prostate cancer management
The use of 18F-FDG PET imaging in prostate cancer depends on the phase of the disease (116). In general, FDG uptake level significantly overlap among normal, benign, and malignant tissues in prostate cancer (116). PET with FDG may be useful in detecting metastasis and treatment response assessment for patients with castrate-resistant metastatic prostate cancer (116). Instead, newer tracers have increased detection accuracies for prostate cancer, including prostate-specific membrane antigen (PSMA)-PET, choline-PET, and 18F-NaF PET/CT (117). Specifically, choline PET/CT has high sensitivity but suffers from low sensitivity, especially at low PSA levels. Nevertheless, choline PET/CT was found to significantly improve upon conventional imaging modalities in the detection of metastatic lesions at biochemical recurrence (117). And PSMA-targeted radiotracers have preliminarily demonstrated great promise in primary and recurrent staging of prostate cancer (117).
Conclusions
Metabolic reprogramming is critical for tumorigenesis and cancer cell adaptation. But cancer metabolic reprogramming is highly heterogeneous and dynamic. Therefore, a deep understanding of cancer metabolic reprogramming is required to target cancer metabolism for precise medicine. To cancer therapy, targeting metabolic reprogramming provides three major approaches to help fight against cancer (Figure 1). (I) Metabolic anti-cancer drugs to target key enzymes or regulators of hyperactivated metabolic pathways (Figure 2). For example, glutamate metabolism is a promising anticancer therapeutic target with glutaminase inhibitors, BPTES and CB839, show great anticancer effect in several tumor models (118). (II) Metabolic modulators to synthesize chemotherapy, radiotherapy and immunotherapy. (III) Dietary interventions to suppress cancer progression (Figure 3). While to cancer monitoring, rewiring cancer metabolism enables metabolic imaging to detect tumor early response to different anti-cancer therapies. And accurate cancer subtyping based on metabolic pathway alterations help select beneficiaries to anti-cancer drugs. For instance, on the basis of metabolic pathways, triple negative breast cancer cells can be classified into the lipogenic subtype with upregulated lipid metabolism, the glycolytic subtype with upregulated carbohydrate and nucleotide metabolism, and mixed subtype (119). Interestingly, three subtypes show distinct sensitivities to various metabolic inhibitors, and inhibition of lactate dehydrogenase could enhance the anti-PD-1 immunotherapy response in the glycolytic subtype of TNBC (119). Therefore, integration of metabolic characteristic and metabolic imaging could guide the precise usage of anti-cancer drugs.
Despite of the achievements about targeting cancer metabolism mentioned above, cancer metabolic heterogeneity need be carefully considered when targeting cancer metabolism for clinical translation. Moreover, metabolic dynamic changes are ignorable during tumor progression, accompanying with genetical dynamic changes. Increasing studies have shown that the process of metabolic reprogramming is highly dynamic (2). Furthermore, identification of metabolic checkpoints between normal cells and cancer cells with various genetic mutations is essential for reducing toxic side effects of metabolic anti-cancer strategies. Further research and deeper understanding on the metabolic network and dynamic regulation are required to enhance the implication of cancer metabolism. These insights have the potential to provide new effective therapeutic and monitoring approaches for cancer precision medicine.
Acknowledgments
Funding: This work is supported by a grant to ST from the National Natural Science Foundation of China (82072695).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Zhi-Ming Shao and Xiao-Mao Guo) for the series “Eastern and Western Perspectives on Precision Cancer Medicine” published in Precision Cancer Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at: https://dx.doi.org/10.21037/pcm-21-27
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/pcm-21-27). The series “Eastern and Western Perspectives on Precision Cancer Medicine” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects for the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Faubert B, Solmonson A, DeBerardinis RJ. Metabolic reprogramming and cancer progression. Science 2020;368:eaaw5473 [Crossref] [PubMed]
- Fendt SM, Frezza C, Erez A. Targeting Metabolic Plasticity and Flexibility Dynamics for Cancer Therapy. Cancer Discov 2020;10:1797-807. [Crossref] [PubMed]
- Damaghi M, West J, Robertson-Tessi M, et al. The harsh microenvironment in early breast cancer selects for a Warburg phenotype. Proc Natl Acad Sci U S A 2021;118:e2011342118 [Crossref] [PubMed]
- Warburg O, Wind F, Negelein E. THE METABOLISM OF TUMORS IN THE BODY. J Gen Physiol 1927;8:519-30. [Crossref] [PubMed]
- Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell 2012;21:297-308. [Crossref] [PubMed]
- Kato Y, Ozawa S, Miyamoto C, et al. Acidic extracellular microenvironment and cancer. Cancer Cell Int 2013;13:89. [Crossref] [PubMed]
- Faubert B, Li KY, Cai L, et al. Lactate Metabolism in Human Lung Tumors. Cell 2017;171:358-371.e9. [Crossref] [PubMed]
- Hui S, Ghergurovich JM, Morscher RJ, et al. Glucose feeds the TCA cycle via circulating lactate. Nature 2017;551:115-8. [Crossref] [PubMed]
- Zhang W, Wang G, Xu ZG, et al. Lactate Is a Natural Suppressor of RLR Signaling by Targeting MAVS. Cell 2019;178:176-189.e15. [Crossref] [PubMed]
- Colegio OR, Chu NQ, Szabo AL, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 2014;513:559-63. [Crossref] [PubMed]
- Lai JH, Jan HJ, Liu LW, et al. Nodal regulates energy metabolism in glioma cells by inducing expression of hypoxia-inducible factor 1α. Neuro Oncol 2013;15:1330-41. [Crossref] [PubMed]
- Michelakis ED, Sutendra G, Dromparis P, et al. Metabolic modulation of glioblastoma with dichloroacetate. Sci Transl Med 2010;2:31ra34 [Crossref] [PubMed]
- Kelloff GJ, Hoffman JM, Johnson B, et al. Progress and promise of FDG-PET imaging for cancer patient management and oncologic drug development. Clin Cancer Res 2005;11:2785-808. [Crossref] [PubMed]
- Vasan K, Werner M, Chandel NS. Mitochondrial Metabolism as a Target for Cancer Therapy. Cell Metab 2020;32:341-52. [Crossref] [PubMed]
- Weinberg SE, Sena LA, Chandel NS. Mitochondria in the regulation of innate and adaptive immunity. Immunity 2015;42:406-17. [Crossref] [PubMed]
- Samper E, Morgado L, Estrada JC, et al. Increase in mitochondrial biogenesis, oxidative stress, and glycolysis in murine lymphomas. Free Radic Biol Med 2009;46:387-96. [Crossref] [PubMed]
- Yang Y, Karakhanova S, Hartwig W, et al. Mitochondria and Mitochondrial ROS in Cancer: Novel Targets for Anticancer Therapy. J Cell Physiol 2016;231:2570-81. [Crossref] [PubMed]
- Viale A, Pettazzoni P, Lyssiotis CA, et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature 2014;514:628-32. [Crossref] [PubMed]
- Martínez-Reyes I, Cardona LR, Kong H, et al. Mitochondrial ubiquinol oxidation is necessary for tumour growth. Nature 2020;585:288-92. [Crossref] [PubMed]
- Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med 2016;374:311-22. [Crossref] [PubMed]
- DiNardo CD, Pratz KW, Letai A, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol 2018;19:216-28. [Crossref] [PubMed]
- Guièze R, Liu VM, Rosebrock D, et al. Mitochondrial Reprogramming Underlies Resistance to BCL-2 Inhibition in Lymphoid Malignancies. Cancer Cell 2019;36:369-384.e13. [Crossref] [PubMed]
- Xu J, Ji L, Ruan Y, et al. UBQLN1 mediates sorafenib resistance through regulating mitochondrial biogenesis and ROS homeostasis by targeting PGC1β in hepatocellular carcinoma. Signal Transduct Target Ther 2021;6:190. [Crossref] [PubMed]
- Zhang L, Yao Y, Zhang S, et al. Metabolic reprogramming toward oxidative phosphorylation identifies a therapeutic target for mantle cell lymphoma. Sci Transl Med 2019;11:eaau1167 [Crossref] [PubMed]
- Hayes JD, Dinkova-Kostova AT, Tew KD. Oxidative Stress in Cancer. Cancer Cell 2020;38:167-97. [Crossref] [PubMed]
- Canli Ö, Nicolas AM, Gupta J, et al. Myeloid Cell-Derived Reactive Oxygen Species Induce Epithelial Mutagenesis. Cancer Cell 2017;32:869-883.e5. [Crossref] [PubMed]
- van der Reest J, Lilla S, Zheng L, et al. Proteome-wide analysis of cysteine oxidation reveals metabolic sensitivity to redox stress. Nat Commun 2018;9:1581. [Crossref] [PubMed]
- Klaunig JE. Oxidative Stress and Cancer. Curr Pharm Des 2018;24:4771-8. [Crossref] [PubMed]
- Piskounova E, Agathocleous M, Murphy MM, et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 2015;527:186-91. [Crossref] [PubMed]
- Gentric G, Kieffer Y, Mieulet V, et al. PML-Regulated Mitochondrial Metabolism Enhances Chemosensitivity in Human Ovarian Cancers. Cell Metab 2019;29:156-173.e10. [Crossref] [PubMed]
- Marinho HS, Real C, Cyrne L, et al. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol 2014;2:535-62. [Crossref] [PubMed]
- Luo M, Shang L, Brooks MD, et al. Targeting Breast Cancer Stem Cell State Equilibrium through Modulation of Redox Signaling. Cell Metab 2018;28:69-86.e6. [Crossref] [PubMed]
- Lu H, Samanta D, Xiang L, et al. Chemotherapy triggers HIF-1-dependent glutathione synthesis and copper chelation that induces the breast cancer stem cell phenotype. Proc Natl Acad Sci U S A 2015;112:E4600-9. [Crossref] [PubMed]
- Matés JM, Campos-Sandoval JA, Santos-Jiménez JL, et al. Dysregulation of glutaminase and glutamine synthetase in cancer. Cancer Lett 2019;467:29-39. [Crossref] [PubMed]
- Yang L, Venneti S, Nagrath D. Glutaminolysis: A Hallmark of Cancer Metabolism. Annu Rev Biomed Eng 2017;19:163-94. [Crossref] [PubMed]
- Durán RV, Oppliger W, Robitaille AM, et al. Glutaminolysis activates Rag-mTORC1 signaling. Mol Cell 2012;47:349-58. [Crossref] [PubMed]
- Romero R, Sayin VI, Davidson SM, et al. Keap1 loss promotes Kras-driven lung cancer and results in dependence on glutaminolysis. Nat Med 2017;23:1362-8. [Crossref] [PubMed]
- Weinberg F, Hamanaka R, Wheaton WW, et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A 2010;107:8788-93. [Crossref] [PubMed]
- Chen R, Lai LA, Sullivan Y, et al. Disrupting glutamine metabolic pathways to sensitize gemcitabine-resistant pancreatic cancer. Sci Rep 2017;7:7950. [Crossref] [PubMed]
- Kodama M, Oshikawa K, Shimizu H, et al. A shift in glutamine nitrogen metabolism contributes to the malignant progression of cancer. Nat Commun 2020;11:1320. [Crossref] [PubMed]
- Akins NS, Nielson TC, Le HV. Inhibition of Glycolysis and Glutaminolysis: An Emerging Drug Discovery Approach to Combat Cancer. Curr Top Med Chem 2018;18:494-504. [Crossref] [PubMed]
- Yoo HC, Park SJ, Nam M, et al. A Variant of SLC1A5 Is a Mitochondrial Glutamine Transporter for Metabolic Reprogramming in Cancer Cells. Cell Metab 2020;31:267-283.e12. [Crossref] [PubMed]
- Sivanand S, Vander Heiden MG. Emerging Roles for Branched-Chain Amino Acid Metabolism in Cancer. Cancer Cell 2020;37:147-56. [Crossref] [PubMed]
- Mayers JR, Wu C, Clish CB, et al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat Med 2014;20:1193-8. [Crossref] [PubMed]
- Li JT, Yin M, Wang D, et al. BCAT2-mediated BCAA catabolism is critical for development of pancreatic ductal adenocarcinoma. Nat Cell Biol 2020;22:167-74. [Crossref] [PubMed]
- Ananieva EA, Wilkinson AC. Branched-chain amino acid metabolism in cancer. Curr Opin Clin Nutr Metab Care 2018;21:64-70. [Crossref] [PubMed]
- Clare CE, Brassington AH, Kwong WY, et al. One-Carbon Metabolism: Linking Nutritional Biochemistry to Epigenetic Programming of Long-Term Development. Annu Rev Anim Biosci 2019;7:263-87. [Crossref] [PubMed]
- Pan S, Fan M, Liu Z, et al. Serine, glycine and one-carbon metabolism in cancer Int J Oncol 2021;58:158-70. (Review). [Crossref] [PubMed]
- Ulrich CM, Robien K, Sparks R. Pharmacogenetics and folate metabolism -- a promising direction. Pharmacogenomics 2002;3:299-313. [Crossref] [PubMed]
- Lee WD, Pirona AC, Sarvin B, et al. Tumor Reliance on Cytosolic versus Mitochondrial One-Carbon Flux Depends on Folate Availability. Cell Metab 2021;33:190-198.e6. [Crossref] [PubMed]
- Gao X, Sanderson SM, Dai Z, et al. Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature 2019;572:397-401. [Crossref] [PubMed]
- Kalev P, Hyer ML, Gross S, et al. MAT2A Inhibition Blocks the Growth of MTAP-Deleted Cancer Cells by Reducing PRMT5-Dependent mRNA Splicing and Inducing DNA Damage. Cancer Cell 2021;39:209-224.e11. [Crossref] [PubMed]
- Xu Q, Li Y, Gao X, et al. HNF4α regulates sulfur amino acid metabolism and confers sensitivity to methionine restriction in liver cancer. Nat Commun 2020;11:3978. [Crossref] [PubMed]
- Yang M, Vousden KH. Serine and one-carbon metabolism in cancer. Nat Rev Cancer 2016;16:650-62. [Crossref] [PubMed]
- Xia Y, Ye B, Ding J, et al. Metabolic Reprogramming by MYCN Confers Dependence on the Serine-Glycine-One-Carbon Biosynthetic Pathway. Cancer Res 2019;79:3837-50. [Crossref] [PubMed]
- Ngo B, Kim E, Osorio-Vasquez V, et al. Limited Environmental Serine and Glycine Confer Brain Metastasis Sensitivity to PHGDH Inhibition. Cancer Discov 2020;10:1352-73. [Crossref] [PubMed]
- Cissé MY, Pyrdziak S, Firmin N, et al. Targeting MDM2-dependent serine metabolism as a therapeutic strategy for liposarcoma. Sci Transl Med 2020;12:eaay2163 [Crossref] [PubMed]
- Bindea G, Mlecnik B, Tosolini M, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013;39:782-95. [Crossref] [PubMed]
- Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 2010;11:889-96. [Crossref] [PubMed]
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012;21:309-22. [Crossref] [PubMed]
- Kaymak I, Williams KS, Cantor JR, et al. Immunometabolic Interplay in the Tumor Microenvironment. Cancer Cell 2021;39:28-37. [Crossref] [PubMed]
- McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer. Annu Rev Immunol 2019;37:457-95. [Crossref] [PubMed]
- Leone RD, Zhao L, Englert JM, et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science 2019;366:1013-21. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014;515:558-62. [Crossref] [PubMed]
- Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 2015;372:2521-32. [Crossref] [PubMed]
- Lv H, Lv G, Chen C, et al. NAD+ Metabolism Maintains Inducible PD-L1 Expression to Drive Tumor Immune Evasion. Cell Metab 2021;33:110-127.e5. [Crossref] [PubMed]
- Devalaraja S, To TKJ, Folkert IW, et al. Tumor-Derived Retinoic Acid Regulates Intratumoral Monocyte Differentiation to Promote Immune Suppression. Cell 2020;180:1098-1114.e16. [Crossref] [PubMed]
- Meynet O, Ricci JE. Caloric restriction and cancer: molecular mechanisms and clinical implications. Trends Mol Med 2014;20:419-27. [Crossref] [PubMed]
- Pietrocola F, Pol J, Vacchelli E, et al. Caloric Restriction Mimetics Enhance Anticancer Immunosurveillance. Cancer Cell 2016;30:147-60. [Crossref] [PubMed]
- Curry NL, Mino-Kenudson M, Oliver TG, et al. Pten-null tumors cohabiting the same lung display differential AKT activation and sensitivity to dietary restriction. Cancer Discov 2013;3:908-21. [Crossref] [PubMed]
- Kalaany NY, Sabatini DM. Tumours with PI3K activation are resistant to dietary restriction. Nature 2009;458:725-31. [Crossref] [PubMed]
- Caffa I, Spagnolo V, Vernieri C, et al. Fasting-mimicking diet and hormone therapy induce breast cancer regression. Nature 2020;583:620-4. [Crossref] [PubMed]
- Di Tano M, Raucci F, Vernieri C, et al. Synergistic effect of fasting-mimicking diet and vitamin C against KRAS mutated cancers. Nat Commun 2020;11:2332. [Crossref] [PubMed]
- Di Tano M, Longo VD. A fasting-mimicking diet and vitamin C: turning anti-aging strategies against cancer. Mol Cell Oncol 2020;7:1791671 [Crossref] [PubMed]
- Nencioni A, Caffa I, Cortellino S, et al. Fasting and cancer: molecular mechanisms and clinical application. Nat Rev Cancer 2018;18:707-19. [Crossref] [PubMed]
- Vernieri C, Ligorio F, Zattarin E, et al. Fasting-mimicking diet plus chemotherapy in breast cancer treatment. Nat Commun 2020;11:4274. [Crossref] [PubMed]
- Klement RJ, Pazienza V. Impact of Different Types of Diet on Gut Microbiota Profiles and Cancer Prevention and Treatment. Medicina (Kaunas) 2019;55:84. [Crossref] [PubMed]
- Tajan M, Vousden KH. Dietary Approaches to Cancer Therapy. Cancer Cell 2020;37:767-85. [Crossref] [PubMed]
- Hopkins BD, Pauli C, Du X, et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature 2018;560:499-503. [Crossref] [PubMed]
- Xia S, Lin R, Jin L, et al. Prevention of Dietary-Fat-Fueled Ketogenesis Attenuates BRAF V600E Tumor Growth. Cell Metab 2017;25:358-73. [Crossref] [PubMed]
- Kang HB, Fan J, Lin R, et al. Metabolic Rewiring by Oncogenic BRAF V600E Links Ketogenesis Pathway to BRAF-MEK1 Signaling. Mol Cell 2015;59:345-58. [Crossref] [PubMed]
- Maddocks ODK, Athineos D, Cheung EC, et al. Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature 2017;544:372-6. [Crossref] [PubMed]
- Vernieri C, Casola S, Foiani M, et al. Targeting Cancer Metabolism: Dietary and Pharmacologic Interventions. Cancer Discov 2016;6:1315-33. [Crossref] [PubMed]
- Cummings NE, Williams EM, Kasza I, et al. Restoration of metabolic health by decreased consumption of branched-chain amino acids. J Physiol 2018;596:623-45. [Crossref] [PubMed]
- Iwasa J, Shimizu M, Shiraki M, et al. Dietary supplementation with branched-chain amino acids suppresses diethylnitrosamine-induced liver tumorigenesis in obese and diabetic C57BL/KsJ-db/db mice. Cancer Sci 2010;101:460-7. [Crossref] [PubMed]
- Eriksson S, Prigge JR, Talago EA, et al. Dietary methionine can sustain cytosolic redox homeostasis in the mouse liver. Nat Commun 2015;6:6479. [Crossref] [PubMed]
- Kanarek N, Petrova B, Sabatini DM. Dietary modifications for enhanced cancer therapy. Nature 2020;579:507-17. [Crossref] [PubMed]
- Hoshiya Y, Kubota T, Matsuzaki SW, et al. Methionine starvation modulates the efficacy of cisplatin on human breast cancer in nude mice. Anticancer Res 1996;16:3515-7. [PubMed]
- Poirson-Bichat F, Gonçalves RA, Miccoli L, et al. Methionine depletion enhances the antitumoral efficacy of cytotoxic agents in drug-resistant human tumor xenografts. Clin Cancer Res 2000;6:643-53. [PubMed]
- Goseki N, Yamazaki S, Shimojyu K, et al. Synergistic effect of methionine-depleting total parenteral nutrition with 5-fluorouracil on human gastric cancer: a randomized, prospective clinical trial. Jpn J Cancer Res 1995;86:484-9. [Crossref] [PubMed]
- Xiao HB, Cao WX, Yin HR, et al. Influence of L-methionine-deprived total parenteral nutrition with 5-fluorouracil on gastric cancer and host metabolism. World J Gastroenterol 2001;7:698-701. [Crossref] [PubMed]
- Rabinovich S, Adler L, Yizhak K, et al. Diversion of aspartate in ASS1-deficient tumours fosters de novo pyrimidine synthesis. Nature 2015;527:379-83. [Crossref] [PubMed]
- Chalishazar MD, Wait SJ, Huang F, et al. MYC-Driven Small-Cell Lung Cancer is Metabolically Distinct and Vulnerable to Arginine Depletion. Clin Cancer Res 2019;25:5107-21. [Crossref] [PubMed]
- Prudner BC, Rathore R, Robinson AM, et al. Arginine Starvation and Docetaxel Induce c-Myc-Driven hENT1 Surface Expression to Overcome Gemcitabine Resistance in ASS1-Negative Tumors. Clin Cancer Res 2019;25:5122-34. [Crossref] [PubMed]
- Carracedo A, Weiss D, Leliaert AK, et al. A metabolic prosurvival role for PML in breast cancer. J Clin Invest 2012;122:3088-100. [Crossref] [PubMed]
- Schafer ZT, Grassian AR, Song L, et al. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature 2009;461:109-13. [Crossref] [PubMed]
- Camarda R, Zhou AY, Kohnz RA, et al. Inhibition of fatty acid oxidation as a therapy for MYC-overexpressing triple-negative breast cancer. Nat Med 2016;22:427-32. [Crossref] [PubMed]
- Zaugg K, Yao Y, Reilly PT, et al. Carnitine palmitoyltransferase 1C promotes cell survival and tumor growth under conditions of metabolic stress. Genes Dev 2011;25:1041-51. [Crossref] [PubMed]
- Pike LS, Smift AL, Croteau NJ, et al. Inhibition of fatty acid oxidation by etomoxir impairs NADPH production and increases reactive oxygen species resulting in ATP depletion and cell death in human glioblastoma cells. Biochim Biophys Acta 2011;1807:726-34. [Crossref] [PubMed]
- Jeon SM, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature 2012;485:661-5. [Crossref] [PubMed]
- Beyaz S, Mana MD, Roper J, et al. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 2016;531:53-8. [Crossref] [PubMed]
- Tucci J, Alhushki W, Chen T, et al. Switch to low-fat diet improves outcome of acute lymphoblastic leukemia in obese mice. Cancer Metab 2018;6:15. [Crossref] [PubMed]
- Carracedo A, Cantley LC, Pandolfi PP. Cancer metabolism: fatty acid oxidation in the limelight. Nat Rev Cancer 2013;13:227-32. [Crossref] [PubMed]
- Mokbel K, Mokbel K. Chemoprevention of Breast Cancer With Vitamins and Micronutrients: A Concise Review. In Vivo 2019;33:983-97. [Crossref] [PubMed]
- Hanley MP, Kadaveru K, Perret C, et al. Dietary Methyl Donor Depletion Suppresses Intestinal Adenoma Development. Cancer Prev Res (Phila) 2016;9:812-20. [Crossref] [PubMed]
- Field MS, Lan X, Stover DM, et al. Dietary Uridine Decreases Tumorigenesis in the ApcMin/+ Model of Intestinal Cancer. Curr Dev Nutr 2018;2:nzy013 [Crossref] [PubMed]
- Yun J, Mullarky E, Lu C, et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science 2015;350:1391-6. [Crossref] [PubMed]
- Klein EA, Thompson IM Jr, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011;306:1549-56. [Crossref] [PubMed]
- Magrì A, Germano G, Lorenzato A, et al. High-dose vitamin C enhances cancer immunotherapy. Sci Transl Med 2020;12:eaay8707 [Crossref] [PubMed]
- O'Connor JP, Aboagye EO, Adams JE, et al. Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol 2017;14:169-86. [Crossref] [PubMed]
- Venneti S, Dunphy MP, Zhang H, et al. Glutamine-based PET imaging facilitates enhanced metabolic evaluation of gliomas in vivo. Sci Transl Med 2015;7:274ra17 [Crossref] [PubMed]
- Márquez J, Alonso FJ, Matés JM, et al. Glutamine Addiction In Gliomas. Neurochem Res 2017;42:1735-46. [Crossref] [PubMed]
- Krall AS, Christofk HR. Rethinking glutamine addiction. Nat Cell Biol 2015;17:1515-7. [Crossref] [PubMed]
- Dunphy MPS, Harding JJ, Venneti S, et al. In Vivo PET Assay of Tumor Glutamine Flux and Metabolism: In-Human Trial of 18F-(2S,4R)-4-Fluoroglutamine. Radiology 2018;287:667-75. [Crossref] [PubMed]
- Jadvar H. Is There Use for FDG-PET in Prostate Cancer? Semin Nucl Med 2016;46:502-6. [Crossref] [PubMed]
- Li R, Ravizzini GC, Gorin MA, et al. The use of PET/CT in prostate cancer. Prostate Cancer Prostatic Dis 2018;21:4-21. [Crossref] [PubMed]
- Xiang Y, Stine ZE, Xia J, et al. Targeted inhibition of tumor-specific glutaminase diminishes cell-autonomous tumorigenesis. J Clin Invest 2015;125:2293-306. [Crossref] [PubMed]
- Gong Y, Ji P, Yang YS, et al. Metabolic-Pathway-Based Subtyping of Triple-Negative Breast Cancer Reveals Potential Therapeutic Targets. Cell Metab 2021;33:51-64.e9. [Crossref] [PubMed]
Cite this article as: Zhang H, Tang S. Metabolic reprogramming and cancer precision medicine: a narrative review. Precis Cancer Med 2021;4:35.

