
Sex-based heterogeneity in non-small cell lung cancer (NSCLC) and response to immune checkpoint inhibitors (ICIs): a narrative review
Introduction
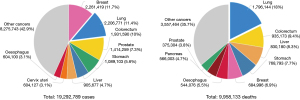
Lung cancer (LC) is still the leading cause of death by cancer worldwide for men and women, with over 2.2 million new cases diagnosed each year (11.4% of total cancer cases) and 1.8 million deaths (18% of total cancer deaths), confirming it the second most frequent cancer and first cause of cancer-related death in men and women combined in 2020 (Figure 1) (1). The 5-year survival of LC reported by the Surveillance, Epidemiology, and End Results (SEER) program in 2011 was 15.6% and in 2019 19.4% (2). Non-small cell lung cancer (NSCLC) accounts for about 85 percent of LCs, with nearly 60% of patients with NSCLC presenting in advanced stages of disease not eligible for radical-intent treatment (1). During the last few decades, management of patients affected by advanced NSCLC (aNSCLC) has dramatically improved mainly due to the introduction of targeted therapies and immune checkpoint inhibitors (ICIs) (3,4). In the setting of non-oncogene addicted aNSCLC, a deeper understanding of the immune cycle control and the discovery of anti-programmed death-1/anti-programmed death-ligand 1 (anti-PD-1/anti-PD-L1) antibodies led to clinically significant improvement in terms of survival, safety and quality of life (5,6). Currently, potential markers for individual prediction of immunotherapy effectiveness lacked high sensitivity and specificity. The selection of patients who could most benefit from ICIs remain crucial, as well as the development of combination strategies for those one unresponsive or refractory to immunotherapy. Furthermore, the optimal treatment duration of ICIs has yet to be clearly defined (7).
The aim of this review is to analyse sex-based differences in aNSCLC patients in terms of clinical-pathological and molecular features focusing on their impact on immune response and outcome. We present the following article in accordance with the Narrative Review reporting checklist (available at https://dx.doi.org/10.21037/pcm-21-7).
Review methods
An extended review of literature through PubMed was conducted, using the keywords related to patient sex (“sex”, “gender”, “male/female”, “men/women”) and NSCLC and LC epidemiological, etiological, clinical-pathological and molecular features. Data collection has been evaluated in order to delineate differences between men and women, highlighting the available level of evidence, when necessary. In the second part of the review, we looked for potential heterogeneous efficacy of ICIs treatments in men vs. women patients diagnosed with aNSCLC.
Sex-based heterogeneity in NSCLC patients
Epidemiology
The lifetime probability of being diagnosed with malignancies is slightly higher for men (40.1%) than for women (38.7%). Overall, the chance that a man will develop LC in his lifetime is about 1 in 15, while for a woman the risk is about 1 in 17 (8).
Worldwide, female breast cancer is the most commonly diagnosed cancer (11.7% of total cases), closely followed by lung (11.4%). LC is the most common cancer in men with 14.3% of new cases, while the third most common cancer in women with 8.4%, behind breast and colorectal cancers. LC remains the leading cancer killer considering all cancer-related deaths (18%), but among women breast cancer represents the principal cause of cancer death (15.5%) followed by LC (13.7%) (1). Incidence and mortality rates are roughly 2 times higher in men than in women, although the male-to-female ratio varies widely across regions, ranging from 1.2 in Northern America to 5.6 in Northern Africa. LC incidence and mortality rates are 3 to 4 times higher in transitioned countries than in transitioning countries, but this pattern may well change as the tobacco epidemic evolves given that 80% of smokers aged ≥15 years resided in low-income and middle-income countries in 2016 (9). Development of tobacco-control policies has led to a decline in the prevalence of smoke habit earlier in men vs. women, and thus to a continuous decreasing in LC mortality rate, but more pronounced in men vs. women (10,11).
According to the Italian Cancer Registry (AIRTUM), in Italy LC is much more frequent in men with one in 10 vs. one in 35 in women of risk of developing this specific cancer. Lung represents the second most frequent primary cancer site in male patients behind prostate (14.1% of new cases), while the third one in female patients behind breast and colorectum (7.3% of new cases). Moreover, LC is the leading cause of cancer-related death in men (23,928 deaths, 23.9%), while among women it is breast cancer (16.1%), followed by LC (9,976 deaths, 12.5%). Interestingly, among women, the increase in LC incidence is confirmed, probably according to the greater smoking habit of women than in the past: specifically, it has been established a decrease in LC incidence in men (−6.5% compared to 2019) and an increase one in women (+2.5% compared to 2019) (12).
Risk factors
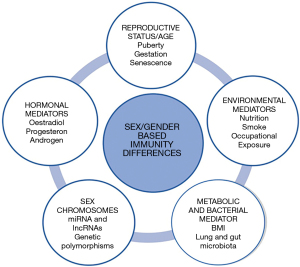
Males and females are possibly differently predisposed to develop LC, due to a series of distinct and/or unbalanced risk factors (Figure 2) (13).
Smoking habit
Tobacco smoke continues to be the primary risk factor for LC development: the chance in long-term smokers has been estimated as 10- to 30-fold compared with never-smokers, moreover risk is proportional to the quantity of cigarette consumption, and important factors include the number of packs per day smoked, the age of onset of smoking, the degree of inhalation, the tar and nicotine content of cigarettes and use of unfiltered cigarettes (14). The World Health Organization (WHO) estimates that 20.2% of the world’s population aged ≥15 years were current smokers in 2015, indicating that smoking rates have decreased by 6.7% globally since 2000 and by 4.1% since 2005. From 2000 onwards, this decreasing trend in smoking rate was registered for both sexes, being faster in men than in women, and equal to −0.22% for year in women and −0.50% for year in men in the period 2010–2015. Nevertheless, smoking remains globally far less common among adult females (6.4%) than among males (34.1%) (9).
A combination of physiological, but also behavioral and cultural factors may contribute to these differences. The counter-proof is represented by the spread of smoking depending on countries. Nowadays female smoking behavior in fact is dominated by higher prevalence in the Americas and European regions, where the differences with males have progressively decreased over time. The prevalence of smoking in American women peaked in 1965 at 33% and begins to slowly decrease only in 1980, while more than half of American men smoked before 1965 with a dramatically decreasing prevalence during the subsequent 20 years (15). Currently, 12.2% of American women smoke cigarettes compared with 15.8% of men (16). Similarly, in Europe the age-standardized prevalence of tobacco smoking has decreased more slowly in women vs. men in the last decades, and forecasts the period 2010–2025 confirm this trend (17).
Among non-smoker patients with LC, women are approximately 20% and seem to prevail over men, with second-hand smoke (SHS) possibly being one of the reasons why. Women living with a smoker partner have a 25–29% increased risk of developing LC (18).
Environmental exposures and diet
Besides tobacco habit, smoking-related lifestyles as well as environmental or occupational exposures are differently expressed between men and women, thus representing possible risk factors for LC related to sex (19-22).
Asbestos, arsenic, radon, polycyclic aromatic hydrocarbons (PAHs), cadmium, nickel, metal dusts and vinyl chloride exposures are recognized as lung carcinogenic (23). PAHs produced by indoor burning of cooking oil and biomass fuels in poorly ventilated areas might be a relevant risk factor for LC. This effect is common among East and South Asian women, but became progressively relevant in all developing countries (24), with lung microbiota (LM) being recognized as a potential etiopathogenetic factor in females LC attributed to household coal burning exposure (25).
Dietary patterns seem to influence LC risk differently by sex. Vitamin C, folate, and carotenoids appear to be protective, while total fat, monounsaturated and saturated fat are associated with LC in men after adjusting for age, education, cigarettes/day, years smoking, and total energy intake (26). Diet did not appear as a major risk factor for LC among women. Nevertheless, in the special subset of never-smoker patients with LC, a sex-independent protective effect was suggested for vegetables/carrots and a deleterious one for cultured milk products, while milk resulted a risk factor only among male high-consumers (27). An inverse association between body mass index (BMI) and LC was observed in men but not in women after adjustment for age and smoking, according to a case-control study based on the results of community mass screening (LC =363, control subjects =1,089) (28). Recent studies showed that certain respiratory microbes and microbiota dysbiosis could correlate with LC development (29), and that sex might influence LM composition after external stimuli exposure (30). However, the possible impact of sex-biased LM in LC risk has to be demonstrated.
DNA adducts and DNA repair systems
Interestingly, sex differences on molecular/genetic levels also suggest a distinctive sensitivity of women toward tobacco-specific carcinogens as compared to men. Tobacco smoke contains a multitude of carcinogens belong to multiple chemical classes, which exert their biologic effect through the formation of DNA adducts in lung tissue. Most carcinogens require a metabolic activation process, generally catalysed by cytochrome P-450 enzymes (P-450s), to oxidize the hydrocarbons, producing reactive oxygen species or intermediates, later neutralized into water soluble conjugates. Reactive intermediates that are not detoxified bind DNA into DNA adducts, playing a role in lung carcinogenesis (31). The balance between metabolic activation and detoxification of carcinogens varies among men and women and may affects LC susceptibility: in particular, it has been hypothesized that women are more susceptible to tobacco carcinogens than men. Estrogen receptors (ERs) are present in both normal and neoplastic lung tissues and could accelerate the metabolism of tobacco carcinogens in a dose-dependent way, as suggested by higher levels of PAH-related DNA adducts in female smokers compared to males (32). Inherited genetic polymorphisms affecting activating and detoxifying enzymes could explain a different susceptibility between sexes to tobacco carcinogens.
Female smokers have a higher expression of cytochrome P-4501A1 (CYP1A1) genes in the lungs than males, resulting in greater carcinogen activation, and this increased expression might be hormone induced (33). A cross-talk between ERs and the aryl hydrocarbon receptor, a regulator of CYP1A1, has been demonstrated in breast cancer cell lines (34). Additionally, several studies have shown that women have higher levels of DNA adducts than men (35). Ryberg et al. have described that among women the DNA adduct levels were higher than in men when adjusted for smoking dose: they found a highly significant difference in the distribution of men and women when smokers were divided into quartile groups according to adducts per pack year, indicating that women are at greater risk of tobacco-induced LC (36). This may confirm that women are at greater risk of tobacco-induced LC.
The most common gene involved in neutralizing adducts is glutathione S-transferase M1 (GTSM1). A GTSM1 homozygous deletion (GTSM1 null) genotype, which is present in 40–60% of the general population, results in the accumulation of free radicals and carcinogenic metabolites. Women exhibit a more prominent polymorphism in GTSM1 null genotype gene deletion induced, which increases the risk for smoking-related cancers (37). Polymerase chain reaction analysis of peripheral blood indicated that women had a greater cancer risk than men [odds ratio (OR), 4.98 vs. 1.37], if they harbor a mutant CYP1A1 genotype. The absence of a functional GTSM1 enzyme alone was not associated with an increased risk of LC, but the CYP1A1 mutation and the GSTM1 null genotype are significantly more frequent in female cancer patients than female controls. The combined variant genotypes conferred an OR of 6.54 for LC in women compared with 2.36 in men. This risk was not affected by age or by smoking history (38,39). Moreover, preclinical data suggest that women have lower DNA repair capacity than men, resulting in a deficit in the DNA repair systems which is associated with an increased risk of LC (40).
Furthermore, relevant mutations which may be caused by tobacco carcinogens were found more frequently in women than in men. Studies have shown that tumor protein p53 (TP53) is mutated in over two-thirds of LCs, and that genetic alteration is more frequently found in female patients (41-43). Moreover, mutations in RAS family genes occur in 35% of patients with LC and in particular aNSCLCs show a mutation rate of 35–50%, that results higher in mucinous lung adenocarcinoma (ADC) (44). The mutations are significantly associated with smoking and the resulting DNA adduct formation. Interestingly, mutations are found more frequently in women and younger patients (45-47).
Several studies have focused on sex differences in LC risk for smoking subjects, but data are inconsistent. Risch et al. has been the first to hypothesize that women, dose for dose, are at higher risk sensitivity than men: it was found that the OR for women was almost three times greater than that for men (27.9 vs. 9.6), when smokers with a 40 pack-year smoking history were compared to non-smokers (48). Then, other case-control and absolute risk cohort studies about sex differences support the theory of higher female susceptibility to tobacco-related LC as well (49-53). On the contrary, other case-control and cohort studies have found either no sex difference or a higher rate ratio among men (54-60). Discrepancies among studies might be linked to variation in study design, the definition of smoking exposure, estimation of risks and the use of never smokers or light smokers as the reference category in the analysis.
Sex hormones
Gubbels Bupp et al. analysed the age- and sex hormone-related changes to innate and adaptive immunity, highlighting their importance in the immune system and the subsequent impact on autoimmunity, cancers, and also on the efficacy of vaccination and cancer immunotherapy. The male higher cancer incidence and mortality before menopause has been at least partially attributed to the protective effect of estrogen, linked to enhanced immunosurveillance, as well as tissue-specific effects (61). The anticarcinogenic and pro-apoptotic effect of estrogen might be the results of the interaction with ERβ isoform and/or a consequence of the blood estrogenic level. Oestrogens modulate immune cell function following a threshold effect: physiologic doses of estrogen (approximately 0.5 nmol/L) stimulate inflammatory cytokine production, but supraphysiologic doses (above 50 nmol/L) can depress immune response. Thus estrogen interaction with anticancer surveillance depends on a series of variables, including patients’ sex, age and their blood levels. Estrogens upregulate the inhibitor signal of PD-1 on effector T cells (Teffs) and CD4+CD25+Foxp3+ regulatory T cells (Tregs), thus contributing to repress antitumor immune responses. Findings from murine melanoma cell lines revealed that sex impacts on tumor immunopathogenesis and immunotherapy responses through differential Treg function and B7-homologue 1 (B7-H1) signaling (62). B7-H1 is a co-signaling molecule abundantly expressed on APCs and other immune cells, that contributes to tumor immune evasion and to induced Treg function. As regard specifically LC, ERs are often expressed by LC cells, thus possibly influencing tumor growth (63). Hormone replacement therapy seems to increase incidence of, and mortality from LC (64), while anti-estrogen use was found to correlate with a reduced risk of LC incidence in women (65). Physiologically blood estrogen level is normally higher in females than males, but ERs are expressed on LC cells of both sexes. However, estrogens seem to activate lung ADC cell lines derived from women, but not from men (66,67). ERα and ERβ are two types of classical ERs, with the latter appearing to be commonest on LC cells (68). The prognostic value of ERα rather than ERβ and of their location on LC cell (cytoplasm/nucleus) remains unsolved, and possibly depending by patient’s sex (63). The role of androgenic steroid on LC carcinogenesis is even more misunderstood.
In a murine lung model, androgens exert their effect by binding on androgen receptor (AR), expressed by type II pneumocytes and bronchial epithelium (69). Androgens altered lung gene expression profiles (GEP), by up-regulating transcripts involved in oxygen transport and down-regulating those responsible for DNA repair and recombination. This cytotoxic effect partially explains the carcinogenic effect of androgens, that might reside also in the immune-suppressive action of testosterone, already demonstrated in different immunological disorders (70). Testosterone level has been found to be associated with LC risk, according to a population-based cohort study on men aged 70–88 years (n=3,635), even after adjustment for smoking status (71). Thus, androgens might promote LC origin, but also be involved in LC progression. It is striking in this sense the finding coming from a retrospective analysis (n=3,018) by which androgen deprivation therapy resulted of benefit on survival after LC diagnosis [hazard ratio (HR), 0.36; P=0.0007] (72).
Clinical-pathological and molecular features
LCs are classified into two major classes: small cell lung carcinoma (SCLC) and NSCLC. The latter, which is the predominant type, includes histologic subtypes such as squamous cell carcinoma (SCC), large cell carcinoma (LCC) and ADC. SCC, SCLC and LCC rates declined since the 1990s for both sexes, but less rapidly among females according to changes in smoking habit and cigarette manufacturing (73). Since then, ADC became the most common subtype of LC both in men and women worldwide (74,75). In last decades in most countries ADC rates remained relatively constant in males, while it increased in females (76,77). The subset of lung ADC once recognized as bronchoalveolar carcinoma (BAC) disproportionately affects women (78) but, since the latest WHO classification of LC discontinued the term BAC in favor of “lepidic” (79), no data is currently available about sex-difference incidence in lepidic ADC subtype; not even any valid information about men-women disparities in other ADC subtypes (acinar, solid, papillary, micropapillary) exists. SCLC and SCC histology are typically linked to a heavy smoke exposure, while approximately 10–15% of lung ADC is diagnosed in never-smokers. Near 50% of women diagnosed with LC are never-smokers compared with 15–20% of men, and this proportion is even higher in Asiatic female population (80-83).
As mentioned above, molecular characterization of LC from women reveals a higher mutational frequency in some driver genes, such as TP53 and KRAS (21,84,85). TP53 alterations are associated with increased cancer risk and earlier age at first-cancer diagnosis for females compared to males; female carriers have a 2.5- to 7-fold higher odds of having cancer than male carriers (86). Moreover, specifically in NSCLC, the frequency of G to T transversion mutation on TP53 is higher among females than males (40% and 25% respectively) (42). LC from smokers shows a distinct TP53 mutation spectrum, such as G to T transversions at codons 157, 158, 179, 248, and 273, which are uncommonly observed in never-smokers (87,88). Indeed, in smokers, 43% of the mutations were G to T transversions, but this number dropped to 13% in never-smokers (89). Another analysis reported that the difference of TP53 mutational spectrum between never-smokers and smokers was detectable only in women (42,90). TP53 mutations in female never-smokers with ADC were predominantly transitions (83%), while they consisted predominantly in transversions (60%) and deletions (20%) in smokers (43). As regard KRAS mutation, Nelson et al. reported a significant association between this genetic alteration and female sex in lung ADC tissue after adjustment for carcinogen exposures [OR, 3.3; 95% confidence interval (CI), 1.3–7.9], with mutations found only in smokers. Authors suggested a possible role of estrogen exposure in either the initiation or the selection of KRAS mutant clones in ADC (45). In addition, a large study by Dogan et al. genotyped 3,026 lung ADCs showing that KRAS G12C, typically associated with smoke habit, was more frequent in women (P=0.007). These women were younger than men with the same mutation (median 65 vs. 69 years old, P=0.0008) and smoked less than men (87). The higher frequency of KRAS G12C in women, their younger age, and lesser smoking history support a higher susceptibility to tobacco carcinogens. More recently KRAS G12C mutation was found to occur more often in Caucasian females than in males with NSCLC (OR, 1.4; 95% CI, 1.3 to 1.6; Q<0.001), and similarly in Asiatic population (OR, 5.2; 95% CI, 1.9 to 17.9; Q=0.01) (91). The detection of KRAS G12C mutation have acquired a therapeutic implication, with encouraging results of targeted therapies against solid tumors harboring this one (92,93).
Generally, the presence of most relevant driver mutations in NSCLCs is more common in never or light smokers and female patients. Tumor molecular characterization and the subsequent identification of driver mutations allow the development of personalized molecular targeted therapies and improvement in NSCLC patients’ outcome and prognosis. Female tumors more frequently carry out targetable alterations, such as mutation of epidermal growth factor receptor (EGFR) (94,95), human epidermal growth factor receptor 2 (HER2) (96) or serine/threonine-protein kinase B-RAF as well as rearrangement of proto-oncogene 1 receptor tyrosine kinase (ROS1) (97,98) and anaplastic lymphoma kinase (ALK) (99), even though some discordant data are available on ALK prevalence in females (100,101).
On the other hand, pathogenic mutations of serine/threonine kinase (STK11), RNA-binding protein 10 (RBM10) and SMARCA4 have been reported more frequently in lung ADC samples from males (102); however, none of these mutations is currently targetable by available drugs.
Tumor mutation burden (TMB) acquired increasing relevance in NSCLC, because of their potential predictive value of response to immunotherapy, being an indirect measure of tumor antigenicity generated by somatic tumor mutations (103). LC has a very high rate of somatic mutations when compared to other tumors; 8.7 mutations per megabase in ADCs and 9.7 in SCC are reported (104). Even if TMB do not correlate with PD-L1 expression, they both emerged as key biomarkers of sensitivity to ICIs.
Sex difference in TMB has already been reported in patients affected by cutaneous melanoma, and a more recent next-generation sequencing (NGS) analysis in NSCLC tumor samples confirmed a lower TMB in females than males. In particular, TMB resulted higher in males and 10-fold higher in smokers than in never-smokers (105,106). This correlates with the consistently lower TMB observed in NSCLC harboring most oncogenic drivers such as alterations of EGFR, ALK, ROS1, BRAF-V600E and MET exon 14 genes, with the exception of BRAF non-V600E and KRAS mutant tumors (107). Wang et al. reported that the predictive value of TMB in LC treated with immunotherapy could be sex-oriented, being more significant in women vs. men (108).
Moreover, males and females own some differences in NSCLC immune-genes (109) and microRNA (miRNA) expression (110), but at the moment it is unknown whether these properties have a predictive/prognostic value.
Immune system and tumor microenvironment (TME)
The immune system differs between males and females: women have stronger innate and adaptive (humoral and cellular) immune responses when compared to men, and this is the results of variety in genetic and epigenetic regulators (sex chromosome), sex hormones (androgens, estradiol and progesterone), microbiome and social factors (smoke and alcohol behaviors) (111).
Females have an immune system that acts predominantly by T helper (CD4+) response, specifically with a humoral response (112). Hormone receptors are present in many cells of immune system: especially ERs are expressed in macrophages, lymphocytes and dendritic cells, while progesterone receptors are also detected in the natural killer cells. On the other side, males’ immune system mainly works through a cytotoxic action, with a higher number of T CD8+ lymphocytes and a lower CD4+/CD8+ ratio than females (113,114). The estrogenic signaling partly contributes to the female greater polarization of macrophages towards those called M2-like (115). These alternatively activated macrophages favor cell proliferation and tissue repair, while M1-like classical activated ones’ express high levels of major histocompatibility complex and pro-inflammatory molecules, playing a central role in cellular death of cancer cells. This could become quite challenging in cancer patients, as macrophages orientation in the host could influence his benefit from a ICIs therapy. Some of the discrepancies in immune responses distinguishing males from females and vice versa may be secondarily to sexual hormones action. A pro-TME (116) may be promoted in females by the redundant pathway of 17β-estradiol (E2), even though a gain in PD-L1 increased expression (117). Th1-derived interferon gamma (IFNγ)+ cytokines response was found to be higher in males than in females, after T cells exposure to sex hormone stimulation (118).
These findings about male vs. female immune compartment do not translate in immediate therapeutic implications for cancer patients, the major limitation is that data derives mainly from healthy patients or from patients affected by non-oncological diseases.
The aforementioned M2-oriented macrophage differentiation tracked down in females has not been confirmed in cancer patients; according to a small experience investigating tumor associated macrophages (TAM) in NSCLC patients, TAM features do not differ by sex (119).
Nor studies about any sex-biased in tumor PD-L1 expression have been conclusive to date. By binding to the Treg receptor PD-1, PD-L1 play a key role in cancer immune cycle by promoting self-tolerance and down-regulation of T-cell inflammatory activity (120). Sex resulted to be not correlated with PD-L1 expression according to a meta-analysis of nine studies involving solid cancer patients (n=1,550) (121), and subsequent experiences agreed in this sense (122-124). A larger and more recent meta-analysis of 52 studies showed male sex as associated with PD-L1 expression (OR, 4.8; 95% CI, 3.2–7.2; P<0.001) (125). Discordant findings came from other studies, by which PD-L1 levels are significantly higher in women vs. men affected by solid tumors, including NSCLC (126-128).
Nevertheless, some experiences suggest possible sex-biased in tumor-infiltrating immune cells (TIICs) composition across solid cancers, including NSCLC (129). Recently, TIICs analyses of samples from advanced melanoma patients, partially exhausted cytotoxic T lymphocytes [i.e., tumor-infiltrating CD8+ T cells expressing high levels of cytotoxic T-lymphocyte antigen 4 (CTLA-4) and PD-1] has been found much more in men vs. women (130).
The increasing interest on this topic led recently to comprehensive analyses from The Cancer Genome Atlas (TCGA), that revealed divergent patterns for sex bias in immune features across multiple cancer types. For example, women with SCC had higher than men levels of biomarkers, including cytolytic activity (CYT), GEP, relative richness of activated CD4+ and CD8+ T cells and T cells, 20 out of 34 immune checkpoints and T cell receptor (TCR) abundance. On the counterpart the aneuploidy scores appear lower than in SCC sample of male patients. Surprisingly, female-bias was observed for both inhibitory checkpoints [PD-1, lymphocyte activating 3 (LAG3), CTLA-4, adenosine A2a receptor (ADORA2A)] and stimulatory immune checkpoints [e.g., tumor necrosis factor receptor superfamily member 4 (TNFRSF4), TNF superfamily member 4 (TNFSF4), inducible co-stimulator (ICOS), tumor necrosis factor receptor superfamily 7 (CD27)]. These findings were validated by the authors through independent dataset, that pointed out a female-biased pattern based on checkpoints [e.g., B and T lymphocyte attenuator (BTLA), cluster of differentiation 80 (CD80)] and immune cell populations (e.g., activated CD4+/CD8+ T cell) (130).
Similarly, a recent systematic review and meta-analysis of transcriptomic studies by Pérez-Díez et al. revealed that about 43% of detected functional alterations caused by lung ADC and associated to immune response are upregulated in females (131). These findings support other studies that showed more powerful innate and adaptive immune responses in women than men, with increased phagocytic activity of neutrophils and macrophages, more efficient antigen presenting cells, differences in lymphocyte subsets and cytokine production (111).
Patients’ sex and ICIs efficacy in NSCLC
Therapy with anti-PD-1/PD-L1 antibodies, which was first approved as second‐line treatment in aNSCLC (132-135), was then extended to first-line treatment. ICI demonstrated its superiority over platinum-doublet chemotherapy (ChT) in untreated patients with aNSCLC and high PD-L1 expression (tumor proportion score ≥50%) (136-138); moreover, the combination of pembrolizumab or atezolizumab with ChT showed better activity than ChT alone, regardless of PD-L1 status and histology (139-141).
Actually, the identification of predictive biomarkers for tumor response to immunotherapies is extensively studied in order to improve patient selection and ensure an effective personalized approach. Despite being a useful biomarker, PD-L1 expression by itself is not enough, as other immunologic or non-immunologic markers may influence ICIs efficacy (142). Sex differences could alter the mechanism of immune response modulation, but usually patient’s sex is not considered as a stratification criterion in randomized clinical trials. Data about ICI efficacy in men and women in fact mainly derive from post-hoc subgroup analysis (Table 1).
Table 1
| Trial | Authors | Histology | Stage | Inclusion criteria | Treatment | Comparator | Line | M | F | M to F ratio | PFS, ITT population HR | PFS, male HR (95% CI) | PFS, female HR (95% CI) | OS, ITT population HR | OS, male HR (95% CI) | OS, female HR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CheckMate 057 | Borghaei et al. | nSCC, NSCLC | IIIB–IV | – | Nivolumab | Docetaxel | ≥2 | 319 | 263 | 1.21 | 0.91 | 0.81 (0.63–1.04) | 1.04 (0.80–1.37) | 0.73 | 0.73 (0.56–0.96) | 0.78 (0.58–1.04) |
| CheckMate 017 | Brahmer et al. | SCC, NSCLC | IIIB–IV | – | Nivolumab | Docetaxel | 2 | 208 | 64 | 3.25 | 0.62 | 0.63 (0.46–0.85) | 0.71 (0.40–1.26) | 0.59 | 0.57 (0.41–0.78) | 0.67 (0.36–1.25) |
| KEYNOTE 024 | Reck et al. | NSCLC | IIIB–IV | PD-L1 >50% | Pembrolizumab | Platinum-based ChT | 1 | 187 | 118 | 1.58 | 0.50 | 0.39 (0.26–0.58) | 0.75 (0.46–1.21) | 0.60 | 0.54 (0.36–0.80) | 0.96 (0.56–1.64) |
| KEYNOTE 010 | Herbst et al. | NSCLC | IIIB–IV | PD-L1 ≥1% | Pembrolizumab 2 mg/kg | Docetaxel | 2 | 421 | 266 | 1.58 | 0.88 | 0.78 (0.64–0.94) | 1.02 (0.78–1.32) | 0.71 | 0.65 (0.52–0.81) | 0.69 (0.51–0.94) |
| KEYNOTE 010 | Herbst et al. | NSCLC | IIIB–IV | PD-L1 ≥% | Pembrolizumab 10 mg/kg | Docetaxel | 2 | 422 | 267 | 1.58 | 0.79 | 0.78 (0.64–0.94) | 1.02 (0.78–1.32) | 0.61 | 0.65 (0.52–0.81) | 0.69 |
| OAK | Rittmeyer et al. | NSCLC | IIIB–IV | – | Atezolizumab | Docetaxel | ≥2 | 520 | 330 | 1.58 | 0.93 | NA | NA | 0.75 | 0.81 (0.66–0.99) | 0.66 (0.51–0.86) |
| PACIFIC | Antonia et al. | NSCLC | III unresectable | – | Durvalumab | Placebo | Consolidation after CRT | 500 | 213 | 2.35 | 0.55 | 0.56 (0.44–0.71) | 0.54 (0.37–0.79) | 0.68 | 0.78 (0.59–1.03) | 0.46 (0.30–0.73) |
| KEYNOTE 407 | Paz-Ares et al. | SCC, NSCLC | IIIB–IV | – | ChT + pembrolizumab | Platinum-based ChT | 1 | 455 | 104 | 4.38 | 0.56 | 0.58 (0.66–0.73) | 0.49 (0.3–0.81) | 0.64 | 0.69 (0.51–0.94) | 0.42 (0.22–0.81) |
| KEYNOTE 189 | Gandhi et al. | nSCC, NSCLC | IIIB–IV | – | ChT + pembrolizumab | Platinum-based ChT + pemetrexed | 1 | 363 | 253 | 1.43 | 0.52 | 0.66 (0.5–0.87) | 0.40 (0.29–0.54) | 0.49 | 0.70 (0.50–0.99) | 0.29 (0.19–0.44) |
| IMpower 130 | Cappuzzo et al. | nSCC, NSCLC | IIIB–IV | – | ChT + atezolizumab | Platinum-based ChT | 1 | 400 | 279 | 1.43 | 0.64 | 0.67 (0.54–0.85) | 0.59 (0.45–0.78) | 0.79 | 0.87 (0.66–1.15) | 0.66 (0.46–0.93) |
| IMpower 131 | Jotte et al. | SCC, NSCLC | IIIB–IV | – | ChT + atezolizumab | Platinum-based ChT | 1 | 557 | 126 | 4.42 | 0.71 | 0.71 (0.59–0.85) | 0.66 (0.45–0.97) | 0.88 | 0.91 (0.75–1.12) | 0.68 (0.44–1.04) |
| IMpower 132 | Papadimitrakopoulou et al. | nSCC, NSCLC | IIIB–IV | – | ChT + atezolizumab | Platinum-based ChT + pemetrexed | 1 | 384 | 194 | 1.98 | 0.60 | 0.64 (0.51–0.79) | 0.51 (0.36–0.71) | 0.81 | NA | NA |
| IMpower 150 | Socinski et al. | nSCC, NSCLC | IIIB–IV | Include also EGFR/ALK positive TKI pre-treated | ChT + bevacizumab + atezolizumab | Platinum-based ChT + bevacizumab | 1st ChT | 425 | 267 | 1.59 | 0.62 | 0.55 (0.44–0.67) | 0.73 (0.54–0.96) | 0.78 | NA | NA |
| CheckMate 227 | Hellmann et al. | NSCLC | IIIB–IV | PD-L1 ≥1% | Nivolumab + ipilimumab | Platinum-based ChT | 1 | 515 | 278 | 1.85 | 0.82 | NA | NA | 0.79 | 0.75 (0.61–0.93) | 0.91 (0.69–1.21) |
| CheckMate 227 | Hellmann et al. | NSCLC | IIIB–IV | PD-L1 <1% | Nivolumab + ipilimumab | Platinum-based ChT | 1 | 263 | 110 | 2.39 | 0.75 | NA | NA | 0.62 | 0.55 (0.41–0.73) | 0.83 (0.54–1.28) |
The table reports pivotal trials highlighting the difference between males and females in terms of number-rate of enrolled patients as well as of relative survival benefit. NSCLC, non-small cell lung cancer; ICI, immune checkpoint inhibitor; M, men; F, female; PFS, progression-free survival; OS, overall survival; ITT, intention-to-treat; HR, hazard ratio; CI, confidence interval; nSCC, non-squamous cell carcinoma; SCC, squamous cell carcinoma; PD-L1, programmed death ligand-1; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; TKI, tyrosine kinase inhibitor; ChT, chemotherapy; CRT, chemo-radiotherapy; NA, not assessed.
Recently some oncologist groups specifically investigated the correlation between sex and survival benefit from ICI. According to a five RCTs meta-analysis (n=3,025) comparing a PD-1/PD-L1 agent with docetaxel in ChT-pretreated NSCLC patients, benefit from ICI resulted similar in men (HR, 0.69) compared to women (HR, 0.70); interaction, P=0.82) (143).
Another meta-analysis (n=20 RCTs) showed a greater benefit from ICIs in men than in women across different forms of advanced solid cancers. Considering the pooled HR of the six RCTs that enrolled NSCLC patients (n=3,482), this sex-dependent magnitude of benefit from ICI seemed confirmed even for LC (male pooled HR, 0.72; 95% CI, 0.61–0.86; female pooled HR, 0.89; 95% CI, 0.71–1.11; Pheterogeneity=0.72) (144).
According to a larger systematic review of advanced cancers studies (n=23), sex was found as not associated with efficacy in terms of OS, nor in the NSCLC subgroup analyses that involved eleven RCTs and more than 6,000 patients (male HR, 0.79; 95% CI, 0.71–0.88; female HR, 0.72; 95% CI, 0.56–0.93; P=0.79) (145). Conforti et al. excluded anti-PD-L1 trials in their study, whereas trials with anti-PD-L1 agents were considered in the analysis by Wallis et al. and in a second larger one (n=34) by Yang et al., which pointed out how sex is not associated with cancer immunotherapy survival benefit (146).
Other two meta-analyses performed by Conforti et al. assessed differences in terms of efficacy of the combination of an anti-PD-1/PD-L1 + ChT according to patients’ sex, but focusing on LC patients (147).
The authors examined 8 RCTs reporting outcome of the association of ICI with ChT vs. ChT alone in patients with advanced LC. Results showed that men treated with the combination strategy had a statistically significant reduced risk of progression or death compared with men treated with ChT alone [pooled progression-free survival (PFS): HR, 0.64; 95% CI, 0.58 to 0.71]. While, in women the advantage obtained with immunotherapy + ChT compared with the control arm was larger (pooled PFS: HR, 0.56; 95% CI, 0.49 to 0.65). Interestingly, no statistically significant interaction was found between treatment efficacy and other relevant clinicopathological features, that were age (<65 vs. ≥65 y), smoking status (never vs. former or current smoker), performance status (PS 0 vs. PS 1) and tumor histology.
The second meta-analysis was conducted specifically on RCTs testing an anti-PD-1/PD-L1 given either alone or combined with ChT as front-line systemic treatment for patients with aNSCLC. Analysis considered 3,974 patients, of whom 66.4% were men and 68.9% patients had nonsquamous tumors. Results evidenced that men treated with anti–PD-1 alone had a statistically significantly reduced risk of death as compared with men treated with standard ChT [pooled overall survival (OS): HR, 0.78; 95% CI, 0.60 to 1.00]. On the other side, in women anti-PD-1 alone was not better than standard ChT (pooled OS: HR, 0.97; 95% CI, 0.79 to 1.19). Consistently with previous meta-analysis results, the combination strategy was associated with an OS advantage compared with ChT alone in women, while a statistically significantly smaller benefit was seen in men (female-pooled OS: HR, 0.44; 95% CI, 0.25 to 0.76; male-pooled OS: HR, 0.76; 95% CI, 0.64 to 0.91) (147).
Despite some small reports describing a greater ORR for women vs. men treated with PD-1/PD-L1-inhibitors (130), pivotal trials generally do not report distinctly response rate for patients’ sex. It is not known either if sex could be associated with long-term survival, in the only one trial reporting the sex of patients who survived ≥5 y no apparent difference seems appreciable (M =9; F =7) (148).
Unfortunately, meta-analyses suffer from multiple confounding factors related to disease, treatment option and patient characteristics. RCTs were extremely heterogeneous in terms of included solid tumor types, class and line of ICI and non-ICI therapies. Analyses generally were not performed separately for studies with anti-PD-1 vs. anti-PD-L1 agents, whose action might be differently influenced by patient sexual hormones (149).
Trials allowed enrollment of both untreated and widely pretreated patients, some of the included trials had a ChT at investigator’s choice inside at least one arm, thus amplifying treatments variety. Prognostically this is quite challenging, because antineoplastic agents use variably leads to tumor cellular selection, niche resistance creation and TME properties modulation (150). This heterogeneity compromises the direction and robustness of the results, which are limited also by the poor follow-up time of most considered RCTs. Therefore, the present evidence does not allow to draw any definitive conclusion about patient’s sex and ICI efficacy.
Anyhow, latest research from Conforti et al. offers maybe more concrete insights, being focused on LC and naïve patients (147). As mentioned, women benefited less from single agent ICI vs. ChT, while the advantage of ICI + ChT vs. ChT was shown regardless of patient’s sex, but much more in women. Explanations for this remain speculative, but findings by the same authors about NSCLC TME features help to (151). Tumor samples from women vs. men own greater T-cell dysfunction status, higher expression of inhibitory immune-checkpoint molecules and abundance of immune-suppressive cells thus potentially justifying the impaired efficacy of ICI when administered alone. ChT may subvert these blockades thus eliciting ICI action, more strongly in women vs. men possibly for a difference in neoantigens presentation and immune-evasion mechanisms.
Perspectives and conclusion
Although smoking is the primary risk factor for LC in both men and women, other variable such as genetic differences, sex hormones, environmental exposures and lifestyle habit, immune system and TME disparities, could play an important role in sex-biased carcinogenesis and immune responses (112,152). NSCLC from women have a higher probability of harboring KRAS G12C mutation, that has a negative prognostic value (92).
Several tumor features and response to antineoplastic agents might be sex-dependent. In the latest years, clinicians have tried to find out any predictors of outcome in solid cancers treated with immunotherapy. Patients affected by advanced LC have been investigated too, with some emerging findings about sex differences in ICIs efficacy.
In our opinion, the most interesting finding having a potential impact on clinical practice is that men treated with ICIs alone have a statistically significantly better outcome than women, with these latest benefited more than men by adding ChT agents to ICIs ones instead (147). Associations between female sex and inferior immunotherapy outcomes have also been observed in a real-world retrospective cohort study conducted in the US on aNSCLC patients aged 66 to 89 years (n=19,529) (153).
Results from treatment of cancers other than LC suggested the role of the combination strategy with chemo-immunotherapy that improved survival in female patients. Also in the setting of advanced breast cancer, benefit from immunotherapy alone have been disappointing (154), but, recently, the results of the IMpassion 130 study showed that the combination of atezolizumab + ChT improved outcome compared with ChT alone for women with advanced triple-negative breast cancer, especially in PD-L1 positive patients (155).
Unfortunately, these observations are only suggestive and even for LC patients, data about patients’ sex impact on immunotherapy response remain to date not conclusive and sometimes even discordant. Future studies are warranted to assess the variables sex in an integrated way, taking of care of other known predictive/prognostic factors, in order to determinate the real impact of male and female gender in the field of cancer immunotherapy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Editta Baldini and Franca Melfi) for the series “Lung Cancer In Women: From Epidemiology To Therapy” published in Precision Cancer Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dx.doi.org/10.21037/pcm-21-7
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/pcm-21). The series “Lung Cancer In Women: From Epidemiology To Therapy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Cancer Statistics Review, 1975-2017. Available online: https://seer.cancer.gov/csr/1975_2017/ (accessed 15 February 2021).
- Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature 2018;553:446-54. [Crossref] [PubMed]
- Howlader N, Forjaz G, Mooradian MJ, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med 2020;383:640-9. [Crossref] [PubMed]
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013;39:1-10. [Crossref] [PubMed]
- Onoi K, Chihara Y, Uchino J, et al. Immune checkpoint inhibitors for lung cancer treatment: a review. J Clin Med 2020;9:1362. [Crossref] [PubMed]
- Berghmans T, Durieux V, Hendriks LEL, et al. Immunotherapy: from advanced NSCLC to early stages, an evolving concept. Front Med (Lausanne) 2020;7:90. [Crossref] [PubMed]
- Lung and Bronchus Cancer — Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/lungb.html (accessed 15 February 2021).
- WHO global report on trends in prevalence of tobacco smoking 2000-2025, 2nd ed. Available online: https://apps.who.int/iris/handle/10665/272694 (2018, accessed 15 February 2021).
- Jemal A, Simard EP, Dorell C, et al. Annual Report to the Nation on the Status of Cancer, 1975-2009, featuring the burden and trends in human papillomavirus (HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst 2013;105:175-201. [Crossref] [PubMed]
- Didkowska J, Manczuk M, McNeill A, et al. Lung cancer mortality at ages 35-54 in the European Union: ecological study of evolving tobacco epidemics. BMJ 2005;331:189-91. [Crossref] [PubMed]
- I numeri del cancro in Italia 2020. Available online: https://www.aiom.it/wp-content/uploads/2020/10/2020_Numeri_Cancro-operatori_web.pdf
- Frega S, Dal Maso A, Ferro A, et al. Heterogeneous tumor features and treatment outcome between males and females with lung cancer (LC): Do gender and sex matter? Crit Rev Oncol Hematol 2019;138:87-103. [Crossref] [PubMed]
- U.S. Department of Health and Human Services. Executive Summary (The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General). Available online: https://www.hhs.gov/sites/default/files/consequences-smoking-exec-summary.pdf
- Giovino GA. Epidemiology of tobacco use in the United States. Oncogene 2002;21:7326-40. [Crossref] [PubMed]
- Wang TW, Asman K, Gentzke AS, et al. Tobacco Product Use Among Adults - United States, 2017. MMWR Morb Mortal Wkly Rep 2018;67:1225-32. [Crossref] [PubMed]
- European tobacco use. Available online: http://www.euro.who.int/pubrequest (2019, accessed 15 February 2021).
- North CM, Christiani DC. Women and lung cancer: what is new? Semin Thorac Cardiovasc Surg 2013;25:87-94. [Crossref] [PubMed]
- Kiyohara C, Ohno Y. Sex differences in lung cancer susceptibility: a review. Gend Med 2010;7:381-401. [Crossref] [PubMed]
- Cote ML, Yoo W, Wenzlaff AS, et al. Tobacco and estrogen metabolic polymorphisms and risk of non-small cell lung cancer in women. Carcinogenesis 2009;30:626-35. [Crossref] [PubMed]
- Mollerup S, Berge G, Baera R, et al. Sex differences in risk of lung cancer: Expression of genes in the PAH bioactivation pathway in relation to smoking and bulky DNA adducts. Int J Cancer 2006;119:741-4. [Crossref] [PubMed]
- Subramanian J, Govindan R. Lung cancer in never smokers: a review. J Clin Oncol 2007;25:561-70. [Crossref] [PubMed]
- Kligerman S, White C. Epidemiology of lung cancer in women: risk factors, survival, and screening. AJR Am J Roentgenol 2011;196:287-95. [Crossref] [PubMed]
- Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers--a different disease. Nat Rev Cancer 2007;7:778-90. [Crossref] [PubMed]
- Hosgood HD 3rd, Sapkota AR, Rothman N, et al. The potential role of lung microbiota in lung cancer attributed to household coal burning exposures. Environ Mol Mutagen 2014;55:643-51. [Crossref] [PubMed]
- Bandera EV, Freudenheim JL, Marshall JR, et al. Diet and alcohol consumption and lung cancer risk in the New York State Cohort (United States) Cancer Causes Control 1997;8:828-40. [Crossref] [PubMed]
- Nyberg F, Agrenius V, Svartengren K, et al. Dietary factors and risk of lung cancer in never-smokers. Int J Cancer 1998;78:430-6. [Crossref] [PubMed]
- Kanashiki M, Sairenchi T, Saito Y, et al. Body mass index and lung cancer: a case-control study of subjects participating in a mass-screening program. Chest 2005;128:1490-6. [Crossref] [PubMed]
- Mao Q, Jiang F, Yin R, et al. Interplay between the lung microbiome and lung cancer. Cancer Lett 2018;415:40-8. [Crossref] [PubMed]
- Barfod KK, Vrankx K, Mirsepasi-Lauridsen HC, et al. The murine lung microbiome changes during lung inflammation and intranasal vancomycin treatment. Open Microbiol J 2015;9:167-79. [Crossref] [PubMed]
- Hemminki K. DNA adducts, mutations and cancer. Carcinogenesis 1993;14:2007-12. [Crossref] [PubMed]
- Berge G, Mollerup S. Role of estrogen receptor in regulation of polycyclic aromatic hydrocarbon metabolic activation in lung. Lung Cancer 2004;45:289-97. [Crossref] [PubMed]
- Uppstad H, Osnes GH, Cole KJ, et al. Sex differences in susceptibility to PAHs is an intrinsic property of human lung adenocarcinoma cells. Lung Cancer 2011;71:264-70. [Crossref] [PubMed]
- Thomsen JS, Wang X, Hines RN, et al. Restoration of aryl hydrocarbon (Ah) responsiveness in MDA-MB-231 human breast cancer cells by transient expression of the estrogen receptor. Carcinogenesis 1994;15:933-7. [Crossref] [PubMed]
- Wei Q, Cheng L, Amos CI, et al. Repair of tobacco carcinogen-induced DNA adducts and lung cancer risk: a molecular epidemiologic study. J Natl Cancer Inst 2000;92:1764-72. [Crossref] [PubMed]
- Ryberg D, Hewer A, Phillips DH, et al. Different susceptibility to smoking-induced DNA damage among male and female lung cancer patients. Cancer Res 1994;54:5801-3. [PubMed]
- Nakachi K, Imai K, Hayashi S, et al. Polymorphisms of the CYP1A1 and glutathione S-transferase genes associated with susceptibility to lung cancer in relation to cigarette dose in a Japanese population. Cancer Res 1993;53:2994-9. [PubMed]
- Seidegård J, Pero RW, Markowitz MM, et al. Isoenzyme(s) of glutathione transferase (class Mu) as a marker for the susceptibility to lung cancer: a follow up study. Carcinogenesis 1990;11:33-6. [Crossref] [PubMed]
- Dresler CM, Fratelli C, Babb J, et al. Gender differences in genetic susceptibility for lung cancer. Lung Cancer 2000;30:153-60. [Crossref] [PubMed]
- Garm C, Moreno-Villanueva M, Bürkle A, et al. Age and gender effects on DNA strand break repair in peripheral blood mononuclear cells. Aging Cell 2013;12:58-66. [Crossref] [PubMed]
- Pfeifer GP, Denissenko MF, Olivier M, et al. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene 2002;21:7435-51. [Crossref] [PubMed]
- Kure EH, Ryberg D, Hewer A, et al. p53 mutations in lung tumours: relationship to gender and lung DNA adduct levels. Carcinogenesis 1996;17:2201-5. [Crossref] [PubMed]
- Toyooka S, Tsuda T, Gazdar AF. The TP53 gene, tobacco exposure, and lung cancer. Hum Mutat 2003;21:229-39. [Crossref] [PubMed]
- Lee YS, Bae SC. How do K-RAS-activated cells evade cellular defense mechanisms? Oncogene 2016;35:827-32. [Crossref] [PubMed]
- Nelson HH, Christiani DC, Mark EJ, et al. Implications and prognostic value of K-ras mutation for early-stage lung cancer in women. J Natl Cancer Inst 1999;91:2032-8. [Crossref] [PubMed]
- Soung YH, Lee JW, Kim SY, et al. Mutational analysis of EGFR and K-RAS genes in lung adenocarcinomas. Virchows Arch 2005;446:483-8. [Crossref] [PubMed]
- Matikas A, Mistriotis D, Georgoulias V, et al. Targeting KRAS mutated non-small cell lung cancer: A history of failures and a future of hope for a diverse entity. Crit Rev Oncol Hematol 2017;110:1-12. [Crossref] [PubMed]
- Risch HA, Howe GR, Jain M, et al. Are female smokers at higher risk for lung cancer than male smokers? A case-control analysis by histologic type. Am J Epidemiol 1993;138:281-93. [Crossref] [PubMed]
- Brownson RC, Chang JC, Davis JR. Gender and histologic type variations in smoking-related risk of lung cancer. Epidemiology 1992;3:61-4. [Crossref] [PubMed]
- Harris RE, Zang EA, Anderson JI, et al. Race and sex differences in lung cancer risk associated with cigarette smoking. Int J Epidemiol 1993;22:592-9. [Crossref] [PubMed]
- Zang EA, Wynder EL. Differences in lung cancer risk between men and women: examination of the evidence. J Natl Cancer Inst 1996;88:183-92. [Crossref] [PubMed]
- International Early Lung Cancer Action Program Investigators. Women's susceptibility to tobacco carcinogens and survival after diagnosis of lung cancer. JAMA 2006;296:180-4. [Crossref] [PubMed]
- Tulinius H, Sigfússon N, Sigvaldason H, et al. Risk factors for malignant diseases: a cohort study on a population of 22,946 Icelanders. Cancer Epidemiol Biomarkers Prev 1997;6:863-73. [PubMed]
- Kreuzer M, Müller KM, Brachner A, et al. Histopathologic findings of lung carcinoma in German uranium miners. Cancer 2000;89:2613-21. [Crossref] [PubMed]
- Osann KE, Anton-Culver H, Kurosaki T, et al. Sex differences in lung-cancer risk associated with cigarette smoking. Int J Cancer 1993;54:44-8. [Crossref] [PubMed]
- Simonato L, Agudo A, Ahrens W, et al. Lung cancer and cigarette smoking in Europe: an update of risk estimates and an assessment of inter-country heterogeneity. Int J Cancer 2001;91:876-87. [Crossref] [PubMed]
- Bach PB, Kattan MW, Thornquist MD, et al. Variations in lung cancer risk among smokers. J Natl Cancer Inst 2003;95:470-8. [Crossref] [PubMed]
- Bain C, Feskanich D, Speizer FE, et al. Lung cancer rates in men and women with comparable histories of smoking. J Natl Cancer Inst 2004;96:826-34. [Crossref] [PubMed]
- Freedman ND, Leitzmann MF, Hollenbeck AR, et al. Cigarette smoking and subsequent risk of lung cancer in men and women: analysis of a prospective cohort study. Lancet Oncol 2008;9:649-56. [Crossref] [PubMed]
- De Matteis S, Consonni D, Pesatori AC, et al. Are women who smoke at higher risk for lung cancer than men who smoke? Am J Epidemiol 2013;177:601-12. [Crossref] [PubMed]
- Gubbels Bupp MR, Potluri T, Fink AL, et al. The confluence of sex hormones and aging on immunity. Front Immunol 2018;9:1269. [Crossref] [PubMed]
- Lin PY, Sun L, Thibodeaux SR, et al. B7-H1-dependent sex-related differences in tumor immunity and immunotherapy responses. J Immunol 2010;185:2747-53. [Crossref] [PubMed]
- Schwartz AG, Prysak GM, Murphy V, et al. Nuclear estrogen receptor beta in lung cancer: expression and survival differences by sex. Clin Cancer Res 2005;11:7280-7. [Crossref] [PubMed]
- Chakraborty S, Ganti AK, Marr A, et al. Lung cancer in women: role of estrogens. Expert Rev Respir Med 2010;4:509-18. [Crossref] [PubMed]
- Chu SC, Hsieh CJ, Wang TF, et al. Antiestrogen use in breast cancer patients reduces the risk of subsequent lung cancer: a population-based study. Cancer Epidemiol 2017;48:22-8. [Crossref] [PubMed]
- Dougherty SM, Mazhawidza W, Bohn AR, et al. Gender difference in the activity but not expression of estrogen receptors alpha and beta in human lung adenocarcinoma cells. Endocr Relat Cancer 2006;13:113-34. [Crossref] [PubMed]
- Ivanova MM, Mazhawidza W, Dougherty SM, et al. Sex differences in estrogen receptor subcellular location and activity in lung adenocarcinoma cells. Am J Respir Cell Mol Biol 2010;42:320-30. [Crossref] [PubMed]
- Hsu LH, Chu NM, Kao SH. Estrogen, estrogen receptor and lung cancer. Int J Mol Sci 2017;18:1713. [Crossref] [PubMed]
- Mikkonen L, Pihlajamaa P, Sahu B, et al. Androgen receptor and androgen-dependent gene expression in lung. Mol Cell Endocrinol 2010;317:14-24. [Crossref] [PubMed]
- Trigunaite A, Dimo J, Jørgensen TN. Suppressive effects of androgens on the immune system. Cell Immunol 2015;294:87-94. [Crossref] [PubMed]
- Hyde Z, Flicker L, McCaul KA, et al. Associations between testosterone levels and incident prostate, lung, and colorectal cancer. A population-based study. Cancer Epidemiol Biomarkers Prev 2012;21:1319-29. [Crossref] [PubMed]
- Harlos C, Musto G, Lambert P, et al. Androgen pathway manipulation and survival in patients with lung cancer. Horm Cancer 2015;6:120-7. [Crossref] [PubMed]
- Lewis DR, Check DP, Caporaso NE, et al. US lung cancer trends by histologic type. Cancer 2014;120:2883-92. [Crossref] [PubMed]
- Barta JA, Powell CA, Wisnivesky JP. Global epidemiology of lung cancer. Ann Glob Health 2019;85:8. [Crossref] [PubMed]
- Cheng TY, Cramb SM, Baade PD, et al. The international epidemiology of lung cancer: latest trends, disparities, and tumor characteristics. J Thorac Oncol 2016;11:1653-71. [Crossref] [PubMed]
- Lortet-Tieulent J, Soerjomataram I, Ferlay J, et al. International trends in lung cancer incidence by histological subtype: adenocarcinoma stabilizing in men but still increasing in women. Lung Cancer 2014;84:13-22. [Crossref] [PubMed]
- Meza R, Meernik C, Jeon J, et al. Lung cancer incidence trends by gender, race and histology in the United States, 1973-2010. PLoS One 2015;10:e0121323. [Crossref] [PubMed]
- Raz DJ, He B, Rosell R, et al. Bronchioloalveolar carcinoma: a review. Clin Lung Cancer 2006;7:313-22. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Cufari ME, Proli C, De Sousa P, et al. Increasing frequency of non-smoking lung cancer: Presentation of patients with early disease to a tertiary institution in the UK. Eur J Cancer 2017;84:55-9. [Crossref] [PubMed]
- Viñolas N, Garrido P, Isla D, et al. Lung cancer in never-smoking women: a sub-analysis of the Spanish Female-Specific Database WORLD07. Cancer Invest 2017;35:358-65. [Crossref] [PubMed]
- Wakelee HA, Chang ET, Gomez SL, et al. Lung cancer incidence in never smokers. J Clin Oncol 2007;25:472-8. [Crossref] [PubMed]
- Toh CK, Ong WS, Lim WT, et al. A decade of never-smokers among lung cancer patients-increasing trend and improved survival. Clin Lung Cancer 2018;19:e539-50. [Crossref] [PubMed]
- Foeglé J, Hédelin G, Lebitasy MP, et al. Specific features of non-small cell lung cancer in women: a retrospective study of 1738 cases diagnosed in Bas-Rhin between 1982 and 1997. J Thorac Oncol 2007;2:466-74. [Crossref] [PubMed]
- Barrera-Rodriguez R, Morales-Fuentes J. Lung cancer in women. Lung Cancer (Auckl) 2012;3:79-89. [Crossref] [PubMed]
- Wu CC, Shete S, Amos CI, et al. Joint effects of germ-line p53 mutation and sex on cancer risk in Li-Fraumeni syndrome. Cancer Res 2006;66:8287-92. [Crossref] [PubMed]
- Dogan S, Shen R, Ang DC, et al. Molecular epidemiology of EGFR and KRAS mutations in 3,026 lung adenocarcinomas: higher susceptibility of women to smoking-related KRAS-mutant cancers. Clin Cancer Res 2012;18:6169-77. [Crossref] [PubMed]
- Vähäkangas KH, Bennett WP, Castrén K, et al. p53 and K-ras mutations in lung cancers from former and never-smoking women. Cancer Res 2001;61:4350-6. [PubMed]
- Hernandez-Boussard TM, Hainaut P. A specific spectrum of p53 mutations in lung cancer from smokers: review of mutations compiled in the IARC p53 database. Environ Health Perspect 1998;106:385-91. [Crossref] [PubMed]
- Ding L, Getz G, Wheeler DA, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature 2008;455:1069-75. [Crossref] [PubMed]
- Nassar AH, Adib E, Kwiatkowski DJ. Distribution of KRAS G12C Somatic mutations across race, sex, and cancer type. N Engl J Med 2021;384:185-7. [Crossref] [PubMed]
- Salgia R, Pharaon R, Mambetsariev I, et al. The improbable targeted therapy: KRAS as an emerging target in non-small cell lung cancer (NSCLC). Cell Rep Med 2021;2:100186. [Crossref] [PubMed]
- Hong DS, Fakih MG, Strickler JH, et al. KRASG12C inhibition with sotorasib in advanced solid tumors. N Engl J Med 2020;383:1207-17. [Crossref] [PubMed]
- Zhang YL, Yuan JQ, Wang KF, et al. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget 2016;7:78985-93. [Crossref] [PubMed]
- Yuan Y, Liu L, Chen H, et al. Comprehensive characterization of molecular differences in cancer between male and female patients. Cancer Cell 2016;29:711-22. [Crossref] [PubMed]
- Mazières J, Peters S, Lepage B, et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol 2013;31:1997-2003. [Crossref] [PubMed]
- Sheikine Y, Pavlick D, Klempner SJ, et al. BRAF in lung cancers: analysis of patient cases reveals recurrent BRAF mutations, fusions, kinase duplications, and concurrent alterations. JCO Precis Oncol 2018;2:PO.17.00172.
- Marchetti A, Barberis M, Di Lorito A, et al. ROS1 gene fusion in advanced lung cancer in women: a systematic analysis, review of the literature, and diagnostic algorithm. JCO Precis Oncol 2017;1:1-9. [Crossref]
- Warth A, Penzel R, Lindenmaier H, et al. EGFR, KRAS, BRAF and ALK gene alterations in lung adenocarcinomas: patient outcome, interplay with morphology and immunophenotype. Eur Respir J 2014;43:872-83. [Crossref] [PubMed]
- Paik JH, Choi CM, Kim H, et al. Clinicopathologic implication of ALK rearrangement in surgically resected lung cancer: a proposal of diagnostic algorithm for ALK-rearranged adenocarcinoma. Lung Cancer 2012;76:403-9. [Crossref] [PubMed]
- Fallet V, Cadranel J, Doubre H, et al. Prospective screening for ALK: clinical features and outcome according to ALK status. Eur J Cancer 2014;50:1239-46. [Crossref] [PubMed]
- Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247-53. [Crossref] [PubMed]
- Samstein RM, Lee CH, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 2019;51:202-6. [Crossref] [PubMed]
- Campbell JD, Alexandrov A, Kim J, et al. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat Genet 2016;48:607-16. [Crossref] [PubMed]
- Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013;499:214-8. [Crossref] [PubMed]
- Owada Y, Muto S, Takagi H, et al. Correlation between mutation burden of tumor and immunological/clinical parameters in considering biomarkers of immune checkpoint inhibitors for non-small cell lung cancer (NSCLC). J Clin Oncol 2017;35:abstr e23184.
- Petersen L, Bebb DG. P1.02-013 ATM Mutation as a Predictor for Mutation Burden in NSCLC. J Thorac Oncol 2017;12:S1929. [Crossref]
- Wang S, Zhang J, He Z, et al. The predictive power of tumor mutational burden in lung cancer immunotherapy response is influenced by patients' sex. Int J Cancer 2019;145:2840-9. [Crossref] [PubMed]
- Spigel DR, Schrock AB, Fabrizio D, et al. Total mutation burden (TMB) in lung cancer (LC) and relationship with response to PD-1/PD-L1 targeted therapies. J Clin Oncol 2016;34:abstr 9017.
- Araujo JM, Prado A, Cardenas NK, et al. Repeated observation of immune gene sets enrichment in women with non-small cell lung cancer. Oncotarget 2016;7:20282-92. [Crossref] [PubMed]
- Irelli A, Sirufo MM, D'Ugo C, et al. Sex and Gender Influences on Cancer Immunotherapy Response. Biomedicines 2020;8:232. [Crossref] [PubMed]
- Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016;16:626-38. [Crossref] [PubMed]
- Phiel KL, Henderson RA, Adelman SJ, et al. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol Lett 2005;97:107-13. [Crossref] [PubMed]
- Teilmann SC, Clement CA, Thorup J, et al. Expression and localization of the progesterone receptor in mouse and human reproductive organs. J Endocrinol 2006;191:525-35. [Crossref] [PubMed]
- Hellmann MD, Callahan MK, Awad MM, et al. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell 2018;33:853-61.e4. [Crossref] [PubMed]
- Jackute J, Zemaitis M, Pranys D, et al. Distribution of M1 and M2 macrophages in tumor islets and stroma in relation to prognosis of non-small cell lung cancer. BMC Immunol 2018;19:3. [Crossref] [PubMed]
- Rothenberger NJ, Somasundaram A, Stabile LP. The role of the estrogen pathway in the tumor microenvironment. Int J Mol Sci 2018;19:611. [Crossref] [PubMed]
- Yang L, Huang F, Mei J, et al. Posttranscriptional control of PD-L1 expression by 17β-estradiol via PI3K/Akt signaling pathway in ERα-positive cancer cell lines. Int J Gynecol Cancer 2017;27:196-205. [Crossref] [PubMed]
- Girón-González JA, Moral FJ, Elvira J, et al. Consistent production of a higher TH1:TH2 cytokine ratio by stimulated T cells in men compared with women. Eur J Endocrinol 2000;143:31-6. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014;511:543-50. Erratum in: Nature 2014;514:262 Erratum in: Nature 2018;559:E12. [Crossref] [PubMed]
- Sharpe AH, Wherry EJ, Ahmed R, et al. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol 2007;8:239-45. [Crossref] [PubMed]
- Pan ZK, Ye F, Wu X, et al. Clinicopathological and prognostic significance of programmed cell death ligand1 (PD-L1) expression in patients with non-small cell lung cancer: a meta-analysis. J Thorac Dis 2015;7:462-70. [PubMed]
- Lin G, Fan X, Zhu W, et al. Prognostic significance of PD-L1 expression and tumor infiltrating lymphocyte in surgically resectable non-small cell lung cancer. Oncotarget 2017;8:83986-94. [Crossref] [PubMed]
- Chen Z, Mei J, Liu L, et al. PD-L1 expression is associated with advanced non-small cell lung cancer. Oncol Lett 2016;12:921-7. [Crossref] [PubMed]
- Calles A, Liao X, Sholl LM, et al. Expression of PD-1 and its ligands, PD-L1 and PD-L2, in Smokers and Never Smokers with KRAS-mutant lung cancer. J Thorac Oncol 2015;10:1726-35. [Crossref] [PubMed]
- Thorsson V, Gibbs DL, Brown SD, et al. The Immune Landscape of Cancer. Immunity 2018;48:812-830.e14. [Crossref] [PubMed]
- Loo K, Tsai KK, Mahuron K, et al. Partially exhausted tumor-infiltrating lymphocytes predict response to combination immunotherapy. JCI Insight 2017;2:e93433. [Crossref] [PubMed]
- Conforti F, Pala L. Sex-based heterogeneity of efficacy of anticancer immunotherapy. Ann Oncol 2019;30:v522-3. [Crossref]
- Khan D, Ansar Ahmed S. The Immune System Is a Natural Target for Estrogen Action: Opposing Effects of Estrogen in Two Prototypical Autoimmune Diseases. Front Immunol 2015;6:635. [PubMed]
- Ye Y, Jing Y, Li L, et al. Sex-associated molecular differences for cancer immunotherapy. Nat Commun 2020;11:1779. [Crossref] [PubMed]
- Pérez-Díez I, Hidalgo MR, Malmierca-Merlo P, et al. Functional signatures in non-small-cell lung cancer: a systematic review and meta-analysis of sex-based differences in transcriptomic studies. Cancers (Basel) 2021;13:143. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819-30. [Crossref] [PubMed]
- Herbst RS, Giaccone G, de Marinis F, et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med 2020;383:1328-39. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Rossi G, Russo A, Tagliamento M, et al. Precision medicine for NSCLC in the era of immunotherapy: new biomarkers to select the most suitable treatment or the most suitable patient. Cancers (Basel) 2020;12:1125. [Crossref] [PubMed]
- Lee CK, Man J, Lord S, et al. Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non-small cell lung carcinoma: a systematic review and meta-analysis. JAMA Oncol 2018;4:210-6. [Crossref] [PubMed]
- Conforti F, Pala L, Bagnardi V, et al. Cancer immunotherapy efficacy and patients' sex: a systematic review and meta-analysis. Lancet Oncol 2018;19:737-46. [Crossref] [PubMed]
- Wallis CJD, Butaney M, Satkunasivam R, et al. Association of patient sex with efficacy of immune checkpoint inhibitors and overall survival in advanced cancers: a systematic review and meta-analysis. JAMA Oncol 2019;5:529-36. [Crossref] [PubMed]
- Yang F, Markovic SN, Molina JR, et al. Association of sex, age, and eastern cooperative oncology group performance status with survival benefit of cancer immunotherapy in randomized clinical trials: a systematic review and meta-analysis. JAMA Netw Open 2020;3:e2012534. [Crossref] [PubMed]
- Conforti F, Pala L, Bagnardi V, et al. Sex-based heterogeneity in response to lung cancer immunotherapy: a systematic review and meta-analysis. J Natl Cancer Inst 2019;111:772-81. [Crossref] [PubMed]
- Brahmer J, Horn L, Jackman D, et al. Abstract CT077: Five-year follow-up from the CA209-003 study of nivolumab in previously treated advanced non-small cell lung cancer (NSCLC): Clinical characteristics of long-term survivors. In: Washington, DC: AACR Annual Meeting, 2017. doi:
10.1158/1538-7445.AM2017-CT077 .10.1158/1538-7445.AM2017-CT077 - Pinto JA, Vallejos CS, Raez LE, et al. Gender and outcomes in non-small cell lung cancer: an old prognostic variable comes back for targeted therapy and immunotherapy? ESMO Open 2018;3:e000344. [Crossref] [PubMed]
- Settleman J, Neto JMF, Bernards R. Thinking differently about cancer treatment regimens. Cancer Discov 2021;11:1016-23. [Crossref] [PubMed]
- Conforti F, Pala L, Pagan E, et al. Sex-based dimorphism of anticancer immune response and molecular mechanisms of immune evasion. Clin Cancer Res 2021;27:4311-24. [Crossref] [PubMed]
- Markle JG, Fish EN. SeXX matters in immunity. Trends Immunol 2014;35:97-104. [Crossref] [PubMed]
- Kehl KL, Greenwald S, Chamoun NG, et al. Association between first-line immune checkpoint inhibition and survival for medicare-insured patients with advanced non-small cell lung cancer. JAMA Netw Open 2021;4:e2111113. [Crossref] [PubMed]
- Santa-Maria CA, Nanda R. Immune checkpoint inhibitor therapy in breast cancer. J Natl Compr Canc Netw 2018;16:1259-68. [Crossref] [PubMed]
- Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 2018;379:2108-21. [Crossref] [PubMed]
Cite this article as: Frega S, Ferro A, Bonanno L, Guarneri V, Conte P, Pasello G. Sex-based heterogeneity in non-small cell lung cancer (NSCLC) and response to immune checkpoint inhibitors (ICIs): a narrative review. Precis Cancer Med 2021;4:26.

