
Massive hyper-progression during anti-PD-1 immunotherapy in a young patient with metastatic mucinous adenocarcinoma of the right colon: a case report and literature review
Introduction
Since the first FDA approval of anti-CTLA4 monoclonal antibody (MoAb) ipilimumab for the treatment of metastatic melanoma in 2011 (1), immune checkpoint inhibitors (ICIs) have dramatically changed the treatment landscape of both solid and hematological malignancies. Since them, ICIs as a whole, anti-PD-1 and anti-PD-L1 MoAbs in particular, have become a backbone in the therapeutic armamentarium of melanoma (2,3) as well as of lung (4-10), urothelial (11,12), kidney (13,14), esophageal (15) breast (16), and head and neck cancers (17,18). To date, no biomarker is available which could predict response to ICIs. However, different phenotypical and molecular features were associated with an increased likelihood to benefit from ICIs, regardless of tumor histotype, including high PD-L1 expression, evidence of tumor-infiltrating lymphocytes, and mismatch repair system deficiency (dMMR) (19-21).
Tumor cells harboring dMMR are characterized by high microsatellites instability (MSI-H) and elevated tumor mutational burden (TMB), due to the accumulation of replication errors that confer increased neoantigen load and immunogenicity (22). In this context, studies with ICIs in MSI-H/dMMR tumors showed durable responses to immunotherapy, producing the first FDA agnostic approval of PD-1 inhibitors in this setting (23-25). In metastatic colorectal cancer (mCRC), which was historically considered a non-immunogenic malignancy, the anti-PD-1 MoAb pembrolizumab have also gained recent approval for its use in metastatic MSI-H/dMMR tumors independent from therapy line (26). Similarly, the results of the CheckMate-142 phase II trial (27,28) drove to accelerated FDA regard for nivolumab (anti-PD-1), administered both alone or in combination with low-dose ipilimumab, in pretreated MSI-H/dMMR mCRC, while nivolumab and ipilimumab combination demonstrated robust and durable clinical benefit in the first-line setting (29).
The increasing use of ICIs in clinical practice brought to light novel patterns of response due to their intrinsic mechanism of action. Since immunotherapy works by restoring the host immune response, it is usual to observe delayed radiological responses, after apparent disease progression (30). This phenomenon, referred as “pseudo-progression”, induced to reconsider the classic radiological response (RECIST) criteria to recognize the subgroup of patients benefitting from immunotherapy despite a first apparent lack of response (31-34). Another rare phenomenon linked to ICIs activity is “hyper-progression” (HP), namely a rapid and uncontrollable tumor growth acceleration consequent to immunotherapy, that suggests potentially deleterious effects of these drugs (35). This event was described for the first time by Champiat and colleagues in 2017 (36), who defined HP as a RECIST progression associated with a 2-fold greater increase in tumor growth velocity than before starting immunotherapy. Since then, many other cases of HP have been described in patients affected by melanoma (37,38), renal (39), lung (40), gastric (41,42), neuroendocrine (43), and head and neck tumors (44). By contrast, to our knowledge, only two previous reports of HP have been recorded in mCRC, probably due to the limited number of patients that were previously amenable to ICIs in this setting.
We report a case of an atypical massive progressive disease observed in a young patient with MSI-H mCRC after 2 cycles of up-front pembrolizumab, and provide a literature review of HP under immunotherapy in gastrointestinal malignancies with a particular focus on CRC.
We present the following article in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/pcm-21-10) (45).
Case presentation
In May 2020, a 40-year-old Caucasic man with a medical history of schizoaffective disorder presented to the emergency room of our hospital because of abdominal pain in the right side and low-grade fever for 2 weeks, with increasing dyspnea, asthenia, and loss of appetite. The CT scan showed a large (7.5 cm × 6.5 cm × 8 cm in diameter) abscessed cancer of the right colic flexure, with multiple metastasis in celio-mesenteric, retroperitoneal, axillary and latero-cervical lymph nodes, with no evidence of visceral or skeletal metastases. Due to the life-threatening abscess, patient underwent right hemicolectomy, peri-tumoral lymphadenectomy and ileum-transverse anastomosis with palliative intent. Histological examination revealed a poorly differentiated mucinous adenocarcinoma of the right colon extended to the peritoneal surface (pT4) with endovascular permeation and metastases in 12/20 regional lymph nodes examined (pN2b). Molecular analysis of KRAS, NRAS and BRAF genes did not show any pathogenic mutations. Microsatellite instability was found in 4/7 loci analyzed, thus configuring this tumor as MSI-H. The postoperative course was regular and the patient clinically improved due to regression of fever and dyspnea, while mild pain, asthenia and loss of appetite persisted. The CT scan performed three weeks after surgery confirmed residual nodal disease (2 cm in max diameter), with no evidence of dimensional increase in pre-existing lesions or appearance of new ones. In July 2020, the patient started 1st line immunotherapy with pembrolizumab at the flat dose of 200 mg i.v. (q3w). Concomitant medications were tramadol, megestrol, levosulpiride, as well as antipsychotic drugs used to treat his schizoaffective disorder. A few days after the second dose of pembrolizumab, he rapidly experienced diffuse osteoarticular pain with worsening asthenia, dyspnea and anorexia, thus requiring hospitalization. Blood chemistry tests revealed leukocytosis (15,080 WBC/mcL), anemia (Hb 7.9 g/dL), hypercalcemia (Ca2+ 11.8 mg/dL), and increased levels of C-reactive Protein (CRP, 206 mg/L) and Lactate Dehydrogenase (LDH, 2,118 U/L). Blood culture were negative for infections. Rheumatological tests were normal, thus excluding possible immune-related adverse events (irAE). By contrast, a CT scan evidenced a widespread cancer dissemination for massive bone colonization and new lung metastases, as well numerical and volumetric increase of pre-existing node metastasis (5 cm in max diameter). Figure 1 is representative of the impressive progression occurred between the baseline CT scan and that performed just 8 weeks later, following just 2 cycles of pembrolizumab.
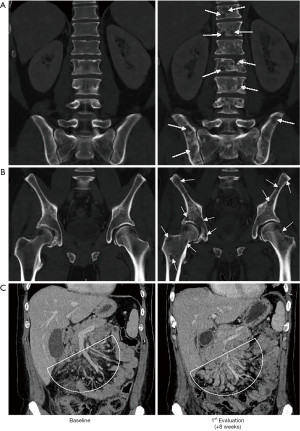
The patient underwent blood transfusions, anti-resorptive therapy with zoledronic acid, as well as analgesic and rehydration therapy as appropriate. Unfortunately, one week after his hospitalization, clinical conditions worsened for disseminated intravascular coagulation (DIC) onset and he died a few days later.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s). The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Discussion
The use of ICIs for the treatment of mCRC proved to be successful in a subset of patients (5%) harboring MSI-H/dMMR tumors. In the recent Keynote-177 phase III trial, first line immunotherapy with pembrolizumab in mCRC almost doubled the percentage of patients free from disease progression at 24 months (48.3%), as compared to standard chemotherapy (18.6%) (46). Although these promising results, almost 40% of patients treated with anti-PD-1 showed an accelerated disease progression as compared to chemo. Although overall survival data are still immature, these observations may suggest a possible detrimental effect of immunotherapy, at least in a sub-set of mCRC patients. However, no valid predictive factor is available in this setting, while sub-group analyses suggested a limited clinical benefit from pembrolizumab only in patients harboring RAS mutated mCRC.
Atypical patterns of response represent another feature of ICIs treatment; one of these is the HP, namely a paradoxical disease acceleration often associated with rapid clinical deterioration. Differently from the “pseudo-progression” that has been widely unraveled, HP lacks strict definition criteria, pathogenic characterization, as well as predictive factors. At a certain extent, it should be interpreted as an immune related adverse event provoked by boosted immune suppression consequent to ICIs administration rather than an accelerated tumor growth. Unfortunately, its timely recognition does not prevent a fatal outcome in the vast majority of patients.
To date, three main studies have tried to define HP; the first one defined HP in the case of a 2-fold greater increase in tumor growth rate (TGR) calculated as a percentage increase in tumor volume over time, particularly within a reference period from 8 weeks before to 8 weeks after treatment start (36). The second definition of HP is based on the time of treatment failure, conventionally set as inferior to 2 months, or on the increase in tumor burden greater than 50% according to the immune-related Response Criteria (irRC) (47). In the third study, the authors defined HP as a 2-fold or greater increase in the TGR on immunotherapy, comparing tumor growth kinetics with one diameter (44). Finally, Matos and colleagues recently proposed a new definition of HP based on RECIST criteria, depending by the presence of at least one of the following conditions: (I) an increment ≥40% in the sum of target lesions as compared to baseline; (II) an increase ≥20% in the sum of target lesions (classic RECIST definition of progressive disease) associated with the emergence of new metastasis in at least two different organs (48). Although all these sound as clear definitions of HP, calculating TGR in clinical practice is complex, while the solely use of RECIST criteria may induce to overestimate HP in patients with intrinsic aggressive disease. Therefore, the recognition of patients experiencing HP remains an unsolved issue.
Different hypotheses have been formulated regarding HP pathogenesis. Several theories agree on the central influence of the immune system, because of its potential in promoting tumor growth by inducing local inflammation and DNA damage. Such tumor microenvironment alterations may work by activating alternative immune checkpoints such as TIM3, by promoting T regulatory cells (Treg) proliferation, or by upregulating pro-inflammatory and pro-tumoral signals (49-55). Moreover, ICIs may also activate immunosuppressive mediators, such as the myeloid derived suppressor cells (MDSCs) and IDO1 (56-58), by increasing IFN-γ levels within the tumor microenvironment, thus contributing to foster immune escape and consequently tumor growth.
Another unmet need is the possibility to identify patients at risk for HP, in order to avoid a potentially deleterious immunotherapy. Clinical factors that have been variously related to the HP included age ≥65 years, poor PS, presence of liver metastases, high tumor burden, as well as increased levels of CRP and neutrophils (49,50). By contrast, no histology-driven mechanisms have been proposed, consistently with a phenomenon mostly described in all tumor subtypes. Recently, Kato and colleagues proposed the MDM2/MDM4 amplification to be predictive of HP (47). They observed that ICIs can increase IFN-γ levels causing the activation of the JAK-STAT pathway. This in turn increases IRF-8 expression that activates MDM2/MDM4 promoters with downstream inhibition of p53 tumor suppressor. It is conceivable that this cascade may not have significant impact when MDM2 is not amplified, but the Authors suppose that it could boost HP in the presence of MDM2 amplification and propose MDM2 inhibitors to counteract this event (51). In addition, Refae and colleagues found a possible correlation of HP with specific single nucleotide polymorphisms (SNPs) of VEGFR1 (rs1870377 A/T or A/A) and PD-L1 (rs2282055 G/T or G/G), although further confirmation is needed (59).
Although continuing case reports of HP suggest that this is not an uncommon phenomenon, its actual incidence is unknown. This in part may be linked to the absence of clear definition criteria. In this context, HP was described in about 4–30% of patients affected by solid tumors treated with anti-PD-1 or anti-PD-L1 MoAbs (60). The incidence, however, seems to be lower in patients treated with anti-CTLA4 (~7%), suggesting a selective anti-PD-1/PD-L1 related event. Moreover, no cases of HP have been reported during chemo-immune combination therapy, probably due to a counterbalancing effect of the cytotoxic drugs (49). Relative to gastrointestinal tumors, the incidence of HP is not well established, accounting <10% of cases. Most of the reports of HP in gastrointestinal cancer regard patients with gastric adenocarcinomas or intestinal neuroendocrine tumors, whereas only few cases regard colorectal tumors. The first report of HP in CRC was described by Zhi Ji (43) in a 31-year-old female with peritoneal metastases from right colon cancer treated up-front with atezolizumab. She experienced progressive disease after one month of therapy for evidence of histologically confirmed dissemination to breast, ovarian, bone and nodes. The second case of HP was reported by Chan (61) in a 48-year-old female affected by Lynch syndrome and right CRC metastatic to the nodes and liver, in progression after two previous treatment lines. Six weeks after the start of anti-PD-1 pembrolizumab, she experienced a 50% size increase of liver and nodal metastases, as well as rapid worsening of clinical conditions that brought the patient to death in few weeks. In line with these two reports, our case concerns a young adult with right sided colon cancer who underwent to massive skeletal colonization and rapid clinical deterioration after just two doses of pembrolizumab. Since no previous evaluation of the disease growth kinetics was calculated, it is difficult to clearly defining as hyper-progression a case presenting with such an advanced disease. However, our patient experienced significant improvement of his systemic symptoms following surgery and post-operative CT scan documented a stable disease almost one month from initial diagnosis. These observations make it reasonable to assume that the disease was in a phase of moderate growth until the start of immunotherapy, while it impressively accelerated with pembrolizumab.
Globally, all these reports may suggest exploring possible relationships between HP and both sidedness and histotype in CRC. Of note, no one of the main definitions of HP currently takes in consideration the evolution of clinical conditions, while it is relevant in our case that clinical deterioration was the first warning of HP. Thus, we argue that rapid modification of physical parameters and clinical worsening from the start of immunotherapy should be included in descriptive criteria for HP definition as it may anticipate the recognition of this phenomenon.
In conclusion, HP is still a not completely understood phenomenon, whose incidence in mCRC is unknown. Despite it has been widely reported in course of immunotherapy, the absence of univocal definition criteria makes not definitive the HP hypothesis in most of cases. Moreover, since predictive factors are not available, its early recognition should be the only way to limit tumor acceleration induced by immunotherapy. Our case, together with the two previous reports in mCRC, claims for adjusting definition criteria of HP as well as continuing efforts in search of putative predictive factors.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/pcm-21-10
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/pcm-21-10). MT declares the following conflict of interest: MSD ACR meeting virtual; NOVARTIS advisory board adjuvant. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517-26. [Crossref] [PubMed]
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med 2019;381:1535-46. [Crossref] [PubMed]
- Robert C, Ribas A, Schachter J, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol 2019;20:1239-51. [Crossref] [PubMed]
- Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819-30. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol 2019;37:537-46. [Crossref] [PubMed]
- Paz-Ares L, Vicente D, Tafreshi A, et al. A Randomized, Placebo-Controlled Trial of Pembrolizumab Plus Chemotherapy in Patients With Metastatic Squamous NSCLC: Protocol-Specified Final Analysis of KEYNOTE-407. J Thorac Oncol 2020;15:1657-69. [Crossref] [PubMed]
- Gadgeel S, Rodríguez-Abreu D, Speranza G, et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol 2020;38:1505-17. [Crossref] [PubMed]
- Gray JE, Villegas A, Daniel D, et al. Three-Year Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC-Update from PACIFIC. J Thorac Oncol 2020;15:288-93. [Crossref] [PubMed]
- Vokes EE, Ready N, Felip E, et al. Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol 2018;29:959-65. [Crossref] [PubMed]
- Horn L, Mansfield AS, Szczęsna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 2018;379:2220-9. [Crossref] [PubMed]
- Fradet Y, Bellmunt J, Vaughn DJ, et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: results of >2 years of follow-up. Ann Oncol 2019;30:970-6. [Crossref] [PubMed]
- Sharma P, Callahan MK, Bono P, et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol 2016;17:1590-8. [Crossref] [PubMed]
- Albiges L, Tannir NM, Burotto M, et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open 2020;5:e001079. [Crossref] [PubMed]
- Powles T, Plimack ER, Soulières D, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol 2020;21:1563-73. [Crossref] [PubMed]
- Kato K, Shah MA, Enzinger P, et al. KEYNOTE-590: Phase III study of first-line chemotherapy with or without pembrolizumab for advanced esophageal cancer. Future Oncol 2019;15:1057-66. [Crossref] [PubMed]
- Schmid P, Rugo HS, Adams S, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2020;21:44-59. [Crossref] [PubMed]
- Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 2019;394:1915-28. [Crossref] [PubMed]
- Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab vs investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol 2018;81:45-51. [Crossref] [PubMed]
- Zito Marino F, Ascierto PA, Rossi G, et al. Are tumor-infiltrating lymphocytes protagonists or background actors in patient selection for cancer immunotherapy? Expert Opin Biol Ther 2017;17:735-46. [Crossref] [PubMed]
- High TMB Predicts Immunotherapy Benefit. Cancer Discov 2018;8:668. [PubMed]
- Goodman AM, Piccioni D, Kato S, et al. Prevalence of PDL1 Amplification and Preliminary Response to Immune Checkpoint Blockade in Solid Tumors. JAMA Oncol 2018;4:1237-44. [Crossref] [PubMed]
- Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med 2018;378:2093-104. [Crossref] [PubMed]
- O'Neil BH, Wallmark JM, Lorente D, et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced colorectal carcinoma. PLoS One 2017;12:e0189848. [Crossref] [PubMed]
- Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol 2016;17:956-65. [Crossref] [PubMed]
- Chung HC, Piha-Paul SA, Lopez-Martin J, et al. Pembrolizumab After Two or More Lines of Previous Therapy in Patients With Recurrent or Metastatic SCLC: Results From the KEYNOTE-028 and KEYNOTE-158 Studies. J Thorac Oncol 2020;15:618-27. [Crossref] [PubMed]
- André T, Shiu KK, Kim TW, et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med 2020;383:2207-18. [Crossref] [PubMed]
- Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182-91. [Crossref] [PubMed]
- Overman MJ, Lonardi S, Wong KYM, et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol 2018;36:773-9. [Crossref] [PubMed]
- Lenz HJ, Lonardi S, Zagonel V, et al. Nivolumab plus low-dose ipilimumab as first-line therapy in microsatellite instability-high/DNA mismatch repair deficient metastatic colorectal cancer: Clinical update. J Clin Oncol 2020;38:abstr 11.
- Jia W, Gao Q, Han A, et al. The potential mechanism, recognition and clinical significance of tumor pseudoprogression after immunotherapy. Cancer Biol Med 2019;16:655-70. [PubMed]
- Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009;15:7412-20. [Crossref] [PubMed]
- Oliver B, Axel H, Katarina L. Adaptation and modification of the immune related response criteria (IRRC): IrRECIST. J Clin Oncol 2014;32:e22121. [Crossref]
- Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017;18:e143-52. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Sabio E, Chan TA. The good, the bad, and the ugly: hyperprogression in cancer patients following immune checkpoint therapy. Genome Med 2019;11:43. [Crossref] [PubMed]
- Champiat S, Dercle L, Ammari S, et al. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin Cancer Res 2017;23:1920-8. [Crossref] [PubMed]
- Schuiveling M, Tonk EHJ, Verheijden RJ, et al. Hyperprogressive disease rarely occurs during checkpoint inhibitor treatment for advanced melanoma. Cancer Immunol Immunother 2021;70:1491-6. [Crossref] [PubMed]
- Yilmaz M, Akovali B. Hyperprogression after nivolumab for melanoma: A case report. J Oncol Pharm Pract 2020;26:244-51. [Crossref] [PubMed]
- Soria F, Beleni AI, D'Andrea D, et al. Pseudoprogression and hyperprogression during immune checkpoint inhibitor therapy for urothelial and kidney cancer. World J Urol 2018;36:1703-9. [Crossref] [PubMed]
- Kas B, Talbot H, Ferrara R, et al. Clarification of Definitions of Hyperprogressive Disease During Immunotherapy for Non-Small Cell Lung Cancer. JAMA Oncol 2020;6:1039-46. [Crossref] [PubMed]
- Sasaki A, Nakamura Y, Mishima S, et al. Predictive factors for hyperprogressive disease during nivolumab as anti-PD1 treatment in patients with advanced gastric cancer. Gastric Cancer 2019;22:793-802. [Crossref] [PubMed]
- Aoki M, Shoji H, Nagashima K, et al. Hyperprogressive disease during nivolumab or irinotecan treatment in patients with advanced gastric cancer. ESMO Open 2019;4:e000488. [Crossref] [PubMed]
- Ji Z, Peng Z, Gong J, et al. Hyperprogression after immunotherapy in patients with malignant tumors of digestive system. BMC Cancer 2019;19:705. [Crossref] [PubMed]
- Saâda-Bouzid E, Defaucheux C, Karabajakian A, et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol 2017;28:1605-11. [Crossref] [PubMed]
- Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Andre T, Shiu K, Kim TW, et al. Pembrolizumab versus chemotherapy for microsatellite instability-high/mismatch repair deficient metastatic colorectal cancer: The phase 3 KEYNOTE-177 study. Presented at: 2020 ASCO Virtual Scientific Program 2020;
- Kato S, Goodman A, Walavalkar V, et al. Hyperprogressors after Immunotherapy: Analysis of Genomic Alterations Associated with Accelerated Growth Rate. Clin Cancer Res 2017;23:4242-50. [Crossref] [PubMed]
- Matos I, Martin-Liberal J, García-Ruiz A, et al. Capturing Hyperprogressive Disease with Immune-Checkpoint Inhibitors Using RECIST 1.1 Criteria. Clin Cancer Res 2020;26:1846-55. [Crossref] [PubMed]
- Frelaut M, du Rusquec P, de Moura A, et al. Pseudoprogression and Hyperprogression as New Forms of Response to Immunotherapy. BioDrugs 2020;34:463-76. [Crossref] [PubMed]
- Denis M, Duruisseaux M, Brevet M, et al. How Can Immune Checkpoint Inhibitors Cause Hyperprogression in Solid Tumors? Front Immunol 2020;11:492. [Crossref] [PubMed]
- Wang X, Wang F, Zhong M, et al. The biomarkers of hyperprogressive disease in PD-1/PD-L1 blockage therapy. Mol Cancer 2020;19:81. [Crossref] [PubMed]
- Akbay EA, Koyama S, Carretero J, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov 2013;3:1355-63. [Crossref] [PubMed]
- Moorman JP, Wang JM, Zhang Y, et al. Tim-3 pathway controls regulatory and effector T cell balance during hepatitis C virus infection. J Immunol 2012;189:755-66. [Crossref] [PubMed]
- Barnaba V, Schinzari V. Induction, control, and plasticity of Treg cells: the immune regulatory network revised? Eur J Immunol 2013;43:318-22. [Crossref] [PubMed]
- Peng W, Liu C, Xu C, et al. PD-1 blockade enhances T-cell migration to tumors by elevating IFN-γ inducible chemokines. Cancer Res 2012;72:5209-18. [Crossref] [PubMed]
- Huang B, Pan PY, Li Q, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res 2006;66:1123-31. [Crossref] [PubMed]
- Spranger S, Spaapen RM, Zha Y, et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med 2013;5:200ra116. [Crossref] [PubMed]
- Baban B, Chandler PR, Sharma MD, et al. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol 2009;183:2475-83. [Crossref] [PubMed]
- Refae S, Gal J, Brest P, et al. Hyperprogression under Immune Checkpoint Inhibitor: a potential role for germinal immunogenetics. Sci Rep 2020;10:3565. [Crossref] [PubMed]
- Borcoman E, Kanjanapan Y, Champiat S, et al. Novel patterns of response under immunotherapy. Ann Oncol 2019;30:385-96. [Crossref] [PubMed]
- Chan KH, Lakkasani S, Ramahi A, et al. Hyperprogressive Disease in an Advanced Stage Colon Cancer Patient on Pembrolizumab: A Case Report. Cureus 2020;12:e7764. [Crossref] [PubMed]
Cite this article as: Guarini C, Todisco A, Tucci M, Porta C, Mannavola F. Massive hyper-progression during anti-PD-1 immunotherapy in a young patient with metastatic mucinous adenocarcinoma of the right colon: a case report and literature review. Precis Cancer Med 2021;4:30.

