
Malignant peritoneal mesothelioma: a narrative review of the rationale for and results of treatment using ultraradical local-regional strategy
Introduction
Probably more than any other invasive abdominal and pelvic malignancy, malignant peritoneal mesothelioma (MPM) is a disease with progression limited to the peritoneal surfaces of the abdomen and pelvis. It may, in a small percentage of patients, invade directly through the diaphragm to metastasize to pleural surfaces. Likewise, a small number of patients are found to have mediastinal invasion through the pericardial sac. Iatrogenic dissemination to the abdominal wall at laparotomy sites is unfortunately too common. However, the cause of death in a great majority of patients is local progression on parietal and visceral surfaces to disrupt gastrointestinal and/or ureteral function. Even in the few patients who develop disease outside of the abdomen and pelvis, it is the intraperitoneal component of their disease that results in their demise (1).
With this unusual natural history, an ultraradical local-regional treatment strategy has evolved. With this approach, a median survival of approximately one year has been greatly expanded to a 70% 20-year survival (2).Success with his aggressive approach to treatment requires knowledgeable patient selection, cytoreductive surgery (CRS), perioperative chemotherapy and long-term intraperitoneal chemotherapy. Recent data suggests that more effective systemic treatments may be emerging (3).
We present the following article in accordance with the Narrative Review Reporting Checklist (available at http://dx.doi.org/10.21037/pcm-21-2).
Methods
The information regarding management of malignant peritoneal mesothelioma (MPM) extended over 3 decades. The study was limited to manuscripts in the English language which were directed toward the ultraradical local-regional approach. The study design included an inquiry into selection factor for treatment. Then a display of the local-regional treatments. A review of recently published results of treatment using this strategy was provided to the reader in the introduction.
Selection factors for long-term success
Patient-related variables
Patient-related variables that can be used as selection factors include performance status, age, gender, histopathology, Ki67 proliferation index, extent of disease as measured by CT, prior surgical score (PSS), extent of prior systemic chemotherapy, and extent of disease at the time of surgery (4). In regards to performance status, this ultraradical local-regional treatment requires approximately 8 months to complete. It is a demanding schedule of treatment for the patient, their family and the caregivers. Some poor performance patients can be nutritionally replenished to allow the CRS to proceed. However, usually the disease progression in the absence of treatment results in a worsening of the patient’s condition. Most commonly patients who present with a poor performance status are placed on systemic chemotherapy with pemetrexed and cisplatin. This group of patients has a guarded prognosis (5).Some will respond for several months with an improved quality of life. The duration of survival is not thought to be prolonged by systemic chemotherapy (6). As discussed later, routine use of neoadjuvant chemotherapy is to be strictly avoided to preserve responses to subsequent perioperative chemotherapy and long-term regional chemotherapy.
Age and gender do have an impact on outcome. Young patients survive longer as do female patients (4,7). However, as far as selection for treatment, those factors are rarely considered. Patients greater than age 75 are usually excluded because of the intensity and duration of the treatment.
Histopathology and its knowledgeable interpretation are extremely important for patient selection. The patients with the most aggressive MPM histologies rarely profit long-term from the ultraradical local-regional strategy. As with many cancers, there is a great diversity in the pace at which the disease is progressing and this rate of disease progression can be estimated by the tumor histology. The sarcomatoid MPM, biphasic (combination of epithelial and sarcomatoid) MPM and poorly differentiated epithelial MPM are usually excluded from the regional treatment strategy (8). The patient may achieve some benefit from CRS plus hyperthermic intraperitoneal chemotherapy (HIPEC) but control of disease is almost universally short-lived. The favorable candidates in whom long-term good results are expected are the epithelial MPM histologies that lack poorly differentiated and deeply invasive components.
The Ki67 proliferation index has been shown by the group at Istituto Tumori in Milan to be of value in selecting patients with MPM for CRS plus HIPEC (9). Confirming this observation, Gilani et al. reporting from the Peritoneal Malignancy Institute in Basingstoke, UK showed that MPM patients with Ki67 proliferation index of <7% had a mean overall survival of 70.0 months compared to an index of ≥7% with survival of 32.8 months. This was highly statistically significant. Gilani and coworkers conclude that histologic classification plus the Ki67 proliferation index can assist in adequate patient selection of MPM patients for CRS and HIPEC (10).
Extent of disease is an important determinant of outcome with MPM. The extent of disease at the time of CRS is estimated by the peritoneal cancer index (PCI) (11). It is not known if the extent of MPM is a determinant of outcome in and of itself (12). Or, is it that PCI has a profound impact on the likelihood of a complete cytoreduction? The completeness of cytoreduction score (CCS) (11) is reliably the most statistically important determinant of outcome of the ultraradical local-regional strategy (12).
Recent clinical research with the preoperative CT evaluation of MPM shows that selected CT findings can reliably estimate the outcome of an individual patient (13). These CT images that predict outcome are referred to as concerning CT features (CCTF). The utility of the CCTF was documented when they were identified on preoperative CT and then correlated statistically with survival. These CT findings predicted outcome not at the time of abdominal exploration as did PCI, not at the time of completion of CRS as did the CCS, but this information was available to the multidisciplinary team (MDT) at the time the patient was being evaluated for treatment recommendations. The two most important selection factors for treatment planning by the MDT is the tumor histopathology and the CCTF.
The CCTF were studied by Sugarbaker, Chang and Jelinek (13). The incidence of the preoperative images and their correlation with survival in 100 MPM patients is shown in Table 1. Surprising, even the smallest amount of pleural effusion, almost always within the right pleural space, indicated a poor prognosis (P<0.0001). Pleural effusion was present in 16% of preoperative CTs. The mesentery infiltrated by mesothelioma was depicted in 41% of patients. An example of this CCTF is shown in Figure 1.When this CCTF was present, survival was significantly reduced (P=0.001). Omental infiltration, if it was extensive, was a prognostic indicator. An example of 3+ omental infiltration is shown in Figure 2. When 2+ or 3+ omental infiltration was present, the survival was significantly reduced with a P=0.0012. Ascites was quantitated. A 2+ or 3+ ascites was present in 43% of patients. When present it indicated a reduced prognosis (P=0.002). Figure 3 shows 3+ ascites in a preoperative evaluation of an MPM patient. The preoperative CT, when interpreted as CCTF, can assess prognosis as accurately as PCI or CCS (13). Radiology is an important selection factor for ultraradical local-regional treatment of MPM.
Table 1
| Concerning CT feature | Incidence (%) | Median survival (months) present/not present | Hazard ratio (95% CI) | P value |
|---|---|---|---|---|
| Obstructed small bowel | 7 | 20/74 | 3.93 (1.73, 8.95) | 0.0011 |
| Clumped small bowel mesentery | 16 | 17/82 | 3.13 (1.69, 5.80) | 0.0003 |
| Mesentery infiltrated | 41 | 25/101 | 2.74 (1.65, 4.55) | 0.0001 |
| Deep pelvis invaded | 21 | 17/74 | 2.04 (1.14, 3.68) | 0.0173 |
| Infiltrated porta hepatis and/or >5 cm mass in lesser omentum | 15 | 25/74 | 2.04 (1.10, 3.79) | 0.0230 |
| Mass >5 cm adjacent jejunum | 15 | 38/82 | 2.88 (1.58, 5.25) | 0.0006 |
| Pleural effusion | 16 | 18/82 | 3.32 (1.86, 5.92) | <0.0001 |
| Omental infiltration 2+ and 3+ | 29 | 24/85 | 2.35 (1.40, 3.93) | 0.0012 |
| Ascites 2+ or 3+ | 43 | 25/101 | 2.66 (1.60, 4.42) | 0.0002 |
| Abdominal mass >5 cm | 5 | 39/72 | 1.64 (0.59, 4.53) | 0.3391 |
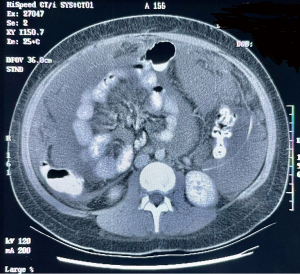
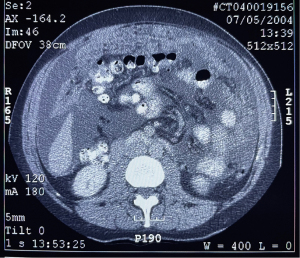
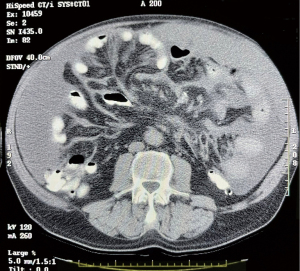
The extent of prior treatment has an impact on benefit expected by ultraradical local-regional treatment. Both the extent of prior surgery and the extent of prior systemic chemotherapy are important. Ideally, the first treatments a MPM patient would receive are those employed by the ultraradical local-regional strategy. Many times, the patient has a prior surgical intervention prior to being referred for definitive treatment of MPM. In women, the presumptive diagnosis for diffuse disease within the abdomen and pelvis is often ovarian cancer. In men, an undiagnosed gastrointestinal cancer, usually suspecting small bowel adenocarcinoma, is the presumptive diagnosis for prior surgical procedures. After extensive surgery the correct histopathologic diagnosis is reported as MPM and the patient is referred to a peritoneal surface malignancy center. The extent of prior surgical treatments is measured by the PSS. The PSS first described by Jacquet et al. quantitates the number of anatomic sites within the abdomen and pelvis that have been dissected or resected in the past (11). The PSS will estimate the adhesive process that the surgeon will encounter during the reoperative event. More importantly, it is an estimate of the extent of tumor cell entrapment that has occurred and will need to be resected as part of a complete CRS. If only a biopsy was performed, the PSS is zero. If there was a prior exploratory laparotomy and a single abdominopelvic region was dissected, the PSS is one. If two to five regions are previously dissected, the PSS is two. If there was a prior attempt at cytoreduction and more than five abdominopelvic regions were involved in the surgical procedure, the PSS is three.
In 2017, we estimated survival of 129 MPM patients treated by CRS and HIPEC (14). When survival by PSS of 0, 1 or ≥2 were compared no statistical significance was present (P=0.6725). However, prior surgery and/or laparoscopy can adversely affect the outcome of patients in other ways. Extensive adhesions will result in a longer and more laborious surgical procedure. In order to completely explore the abdomen and pelvis all intestinal adhesions must be divided. Only after visualization of all parietal and visceral surfaces can the PCI be estimated and the likelihood of a complete CRS be determined. This process can add 2–5 hours to the time required to complete the procedure. Simkens and coworkers from Eindhoven assessed severe morbidity in 211 patients undergoing CRS and HIPEC for colorectal peritoneal metastases (15). Fifty-three patients (25.1%) experienced grade 3 or higher morbidity. The most prominent risk factor for severe morbidity was an extensive prior surgery (odds ratio 4.3). Bekhor and colleagues from New York and Petah Tikva, Israel showed fistulas as a more common late complication of CRS and HIPEC in patients who had a reoperative procedure as compared to the initial CRS and HIPEC (P≤0.01). The reoperative procedure had a greater extent of adhesions and a higher PSS (16). Extensive prior surgery and a high PSS should be avoided by a careful preoperative workup with a high index of suspicion of MPM.
The extent of prior systemic chemotherapy is another variable that can have an impact on prognosis. To date, these effects have not been well studied. Although the clinical impression is strong, little data regarding neoadjuvant systemic chemotherapy and its role in outcome is available. Some oncologists have postulated that neoadjuvant chemotherapy should be used to help select patients with tumors biologically most likely to respond to HIPEC and/or EPIC. There is no doubt that patients who respond to neoadjuvant chemotherapy have a prolonged survival whether or not they have CRS plus perioperative chemotherapy. Others, including myself, have postulated that neoadjuvant systemic treatments will cause apoptosis of responder cancer cells leaving behind a population of cancer cells with natural or acquired resistance. In this situation a profound effect of HIPEC or EPIC would not be expected.
Some data to suggest that neoadjuvant chemotherapy may adversely affect the outcome of CRS plus HIPEC for MPM was provided by a RENAPE multi-institutional retrospective study (3). They collected 126 MPM patients from 20 tertiary centers who had undergone CRS and HIPEC. In their multivariate analysis, neoadjuvant chemotherapy was associated with worse overall survival (P=0.033). They recommended that adjuvant chemotherapy may delay recurrence and improve survival. Upfront CRS and HIPEC should be recommended, if possible.
Currently, until more data becomes available, I recommend the following strategy. If a complete CRS is suggested by the preoperative workup, the initial step in ultraradical local-regional strategy should be a surgical intervention. After CRS plus HIPEC and EPIC, the NIPEC would be administered. If the patient recurs after the treatment plan systemic chemotherapy could be recommended. If the recurrent disease is thought to be resectable to a CC0 or 1, the systemic chemotherapy would follow the second-look procedure (15).
If the preoperative workup by CT or laparoscopy suggests an incomplete CRS, the initial treatment should be neoadjuvant chemotherapy in an attempt to facilitate a CCS of 0 or 1. Le Roy and coworkers working at the Gustave Roussy, Villejuif Cedex, France reported on the use of bidirectional neoadjuvant combined intraperitoneal and intravenous chemotherapy to facilitate conversion surgery (17). They used 2 different preoperative regimens depending on the patient’s prior chemotherapy history. Intraperitoneal pemetrexed was combined with intravenous cisplatin or intraperitoneal oxaliplatin was combined with intravenous gemcitabine. After 3 cycles of bidirectional chemotherapy median laparoscopic PCI was reduced from 27 to 18. Eleven of the 20 patients had a conversion to CRS. There was a 44% 2-year overall survival rate. Intraperitoneal paclitaxel was not used in this study but may be suggested by other reports that attempt conversion surgery (18).
When neoadjuvant systemic chemotherapy precedes CRS, problems are created for which there is no current solutions. First, the true staging of the patient prior to the systemic chemotherapy treatment will never be known. PCI is an important criterion for selection of patients for the ultraradical local-regional strategy; after treatment with neoadjuvant chemotherapy, this variable will never be known. Second, accurate assessment of the extent of resection of tissue that has responded to chemotherapy and no longer appears cancerous by visual inspection is impossible. Biopsies of fibrotic tissues submitted to the pathologist for cryostat section are notoriously unreliable. Usually, the frozen section is negative and the permanent sections with the proper immunostains come back positive. Some advocate resection of all scar tissue because it may be cancer that has responded. This surgical approach is “easier said than done”. The use of neoadjuvant chemotherapy creates a large series of unknowns for complete CRS and should be avoided if at all possible.
The final patient-related selection factor is the PCI. It is a variable of the disease process determined in the operating room. Consequently, its value to the MDT for treatment recommendations is non-existent. A laparoscopic PCI may be useful but the knowledgeable interpretation of the preoperative CT is usually most meaningful. It is a powerful determinant of outcome. Whether in MPM the PCI is an independent prognostic variable in and of itself or it is a predictor of incomplete CCS has not been determined. Sugarbaker and Chang showed in 129 MPM patients that by univariant analysis PCI of <10, 10–30 or >30 showed an impact on survival (P=0.0002) (14). However, on multivariate analysis, the significance was lost (P=0.9026). The complete CRS maintained its significance in the multivariant analysis (P=0.0035). For ovarian cancer, Jónsdóttir and colleagues from Uppsala determined a median PCI of 22 for all patients but median PCI was 33 for patients with an incomplete CRS (19). If patients had a PCI of >24 and a complete CRS, they experienced an increased rate of complications (P=0.008). For ovarian cancer, the PCI seemed to be a predictor of outcome because it determined complete versus incomplete CRS. In contrast, for colorectal cancer, the PCI retains its impact on prognosis in a group of patients with complete CRS (20).
Treatment-related variables
The ultraradical local-regional treatment strategy begins with a complete CRS. By all reports, this is the most important variable regarding a long-term survival in patients treated by this strategy. Of course, resection of a malignancy to no visible evidence of disease will always have a favorable effect on outcome. But also complete (CC0) or near complete cytoreduction (CC1) is required for the other components of this strategy to be effective. The intraperitoneal chemotherapy enters the first several layers of cells surrounding the abdominal and pelvic space by simple diffusion (21). Only a limited penetration of cancer chemotherapy into minute cancer nodules can be expected. Also, a promotion of this tissue penetration over time is required because the capillary network that maintains the viability of the minute cancer nodule will rapidly transfer the cancer chemotherapy from the preperitoneal tissues into the systemic vascular and lymphatic circulations (21).
In our study of 129 patients with MPM treated by CRS and HIPEC, complete CRS was significant in univariant analysis (P<0.0001) and remained significant in the multivariant analysis [hazard ratio 3.98 (1.56–10.13)] with a P value of 0.0038] (14). A complete resection of a malignant process diffusely distributed on most parietal and visceral surfaces requires a series of parietal peritonectomy procedures, visceral resections and a single visceral peritonectomy procedure (22). The complete list of resections that may be required for complete CRS is presented in Table 2. A complete description of these procedures is beyond the scope of this review but an overview is provided.
Table 2
| Peritonectomy | Resections |
|---|---|
| Anterior parietal peritonectomy | Old abdominal incisions, umbilicus, and epigastric fat pad |
| Left upper quadrant peritonectomy | Greater omentectomy and spleen |
| Right upper quadrant peritonectomy | Tumor on Glisson’s capsule of the liver |
| Pelvic peritonectomy | Uterus, ovaries, and rectosigmoid colon |
| Omental bursectomy | Gallbladder and lesser omentum |
| Mesenteric peritonectomy | Right colon and terminal ileum |
Total anterior parietal peritonectomy
With the patient in a modified lithotomy position, an incision is made from xiphoid bone to symphysis pubis. Skin traction sutures are required to elevate the abdominal wall (23). As the peritoneum is dissected away from the posterior rectus sheath using a ball electrosurgical tip, a single entry into the peritoneal cavity in the upper portion of the incision (peritoneal window) allows the surgeon to assess the requirement for a complete anterior parietal peritonectomy (Figure 4). If cancer nodules are palpated on the parietal peritoneum, a complete dissection may be indicated to achieve a complete CRS. If the parietal peritoneum is not involved by MPM, except for the small defect in the peritoneum required for this peritoneal exploration, the remainder of the peritoneum is maintained intact.

The dissecting tool is the ball tip and smoke evacuation is used continuously (24). The peritoneum is most adherent where it directly overlies the transversus muscle. In some instances, dissection from inferior to superior aspects of the abdominal wall facilitates clearing in this area. The dissection blends in with the right and left subphrenic peritonectomy superiorly and with the complete pelvic peritonectomy inferiorly. As the dissection proceeds beyond the peritoneum overlying the paracolic sulcus (line of Toldt), the dissection becomes more rapid with the loose connections of the peritoneum at this anatomic site.
Left subphrenic peritonectomy
Peritonectomy procedures are facilitated by a self-retaining retractor that provides continuous exposure of all quadrants of the abdomen, including the pelvis. Strong traction is exerted on the tumor specimen throughout the left upper quadrant to separate tumor from the diaphragmatic muscle, the left adrenal gland, and the superior half of the perirenal fat. The splenic flexure of the colon is severed from the left abdominal gutter and moved medially by dividing the peritoneum along the line of Toldt (Figure 5). Numerous blood vessels between the diaphragm muscle and its peritoneal surface must be electrocoagulated before their transection so that unnecessary bleeding does not occur as severed blood vessels retract into the muscle of the diaphragm. The plane of dissection is defined using ball-tipped electrosurgery on pure cut, but all blood vessels are electrocoagulated before their division (24).
Right subphrenic peritonectomy
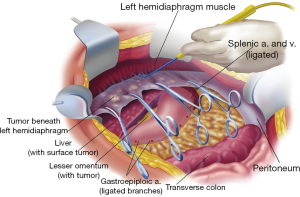
Peritoneum is stripped from beneath the right posterior rectus sheath to begin the peritonectomy in the right upper quadrant of the abdomen. The costal margin is elevated using a self-retaining retractor. Strong traction on the specimen is used to elevate the hemidiaphragm into the operative field. Again, ball-tipped electrosurgery on pure cut is used to dissect at the interface of tumor and normal tissue.
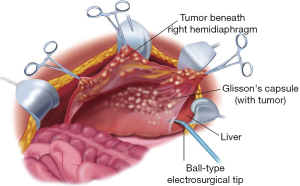
The stripping of tumor from the right hemidiaphragm continues until the bare area of the liver is encountered. At that point, tumor on the superior surface of the liver is electroevaporated until the liver surface is cleared (Figure 6). With ball-tipped electroevaporative dissection, a thick layer of tumor may be bloodlessly lifted off the liver surface by moving beneath Glisson’s capsule using high-voltage, pure-cut electrosurgical dissection. Isolated patches of tumor on the liver surface are electroevaporated with the distal 2 cm of the ball tip bent and stripped of insulation (“hockey-stick” configuration). Ball-tipped electrosurgery is also used to extirpate tumor from attachments of the falciform ligament and round ligament. The dissection continues laterally on the right to encounter the perirenal fat covering the right kidney. The right adrenal gland is visualized and carefully avoided as tumor is stripped from the right subhepatic space. As the peritoneal reflection at the posterior aspect of the liver is divided, care is taken not to traumatize the vena cava or to disrupt the caudate lobe veins that pass between the vena cava and segment 1 of the liver.
Lesser omentectomy and cholecystectomy with stripping of the hepatoduodenal ligament and floor of the omental bursa
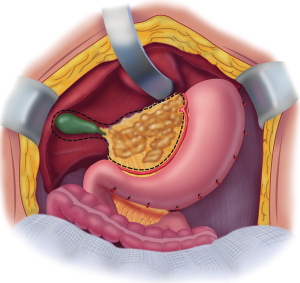
The gallbladder is removed in a routine fashion from its fundus toward the cystic artery and cystic duct; these structures are then ligated and divided. The hepatoduodenal ligament is characteristically heavily layered with tumor. After dividing the peritoneal reflection onto the liver, the cancerous tissue that coats the peritoneal covering of the porta hepatis is bluntly stripped using a Russian forceps from the base of the gallbladder bed toward the duodenum. The right gastric artery going to the lesser omental arcade is preserved. To continue resection of the lesser omentum, the surgeon separates the gastrohepatic ligament from the fissure that divides liver segments 2 and 3 from segment 1. Ball-tipped electrosurgery is used to electroevaporate tumor from the surface of the caudate process. Care is taken not to traumatize the anterior surface of the caudate process, because this can result in excessive and needless blood loss. The segmental blood supply to the caudate lobe is located on the anterior surface of this segment of the liver, and hemorrhage may occur with only superficial trauma. Care must also be taken to avoid an accessory left hepatic artery that may arise from the left gastric artery and cross through the hepatogastric fissure. If the artery is embedded in tumor or its preservation precludes clear exposure of the omental bursa, the artery is ligated as it enters the liver parenchyma. The artery is then resected as part of the hepatogastric ligament.
The triangular ligament of the left lobe of the liver is resected when performing the left subphrenic peritonectomy. After this is completed, the left lateral segment of the liver is retracted left to right to expose the hepatogastric ligament in its entirety. A circumferential electrosurgical release of the lesser omentum from the fissure between liver segments 2 and 3, the left caudate lobe, and the arcade of the right gastric artery to the left gastric artery along the lesser curvature of the stomach is required. After electrosurgically dividing the peritoneum on the lesser curvature of the stomach, digital dissection with extreme pressure from the surgeon’s thumb and index finger separates lesser omental fat and tumor from the vascular arcade (Figure 7), sparing as much of the anterior vagus nerve as possible. The tumor and fatty tissue surrounding the right and left gastric arteries are split from the vascular arcade. In this manner, the specimen is centralized over the major branches of the left gastric artery. With strong traction on the specimen, the lesser omentum is released from the left gastric artery and vein.
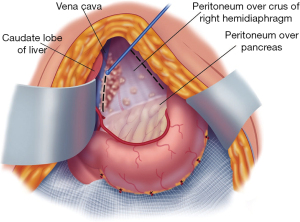
A Deaver retractor or the assistant’s fingertips beneath the left caudate lobe are positioned to expose the entire floor of the omental bursa (Figure 8). Further electroevaporation of tumor from the caudate process of the left caudate lobe of the liver may be necessary to achieve this exposure. Ball-tipped electrosurgery is used to cautiously divide the peritoneal reflection of liver onto the left side of the subhepatic vena cava. After the peritoneum is divided, Russian forceps are used in a blunt stripping of the peritoneum from the superior recess of the omental bursa, from the crus of the right hemidiaphragm, and from beneath the portal vein. Electroevaporation of tumor from the shelf of liver parenchyma beneath the portal vein that connects right and left aspects of the caudate lobe may be required. Care is taken while stripping the floor of the omental bursa to stay superficial to the right phrenic artery.
In some patients, a large volume of tumor on the posterior aspect of the hepatoduodenal ligament may be difficult to visualize. A half-inch Penrose drain placed around the portal triad may allow improved visualization beneath these structures. Use a Russian forceps to tear away the peritoneum beneath the porta hepatis for tumor removal.
Mesenteric peritonectomy
If the mesentery of the ascending colon, transverse colon or descending colon is layered by MPM, it can usually be resected with a sparing of the ileocolic, middle colic and left colic vessels. A layering of tumor on the rectosigmoid colon and rectum usually requires a resection from junction of descending colon and sigmoid colon to the midrectum.
If the mesentery of the small bowel is involved by MPM but the vasculature of the mesentery is spared, a mesenteric peritonectomy procedure is indicated. This peritonectomy was described by Deraco and colleagues (25). The mesenteric peritonectomy starts at the root of the small bowel mesentery and proceeds in a centripetal manner by blunt and electrosurgical dissection to the peritoneal reflection onto the surface of the small bowel.
Complete pelvic peritonectomy with resection of the rectosigmoid colon, uterus and ovaries
The tumor-bearing peritoneum is stripped from the posterior surface of the lower abdominal incision, exposing the rectus muscle. After dissecting generously, the peritoneum on the right and left sides of the bladder, the urachus is localized and placed on strong traction using a Babcock clamp. The peritoneum and underlying fatty tissues are stripped away from the surface of the bladder. Broad traction on the entire anterior parietal peritoneal surface and frequent saline irrigation clears the point for tissue transection, which is precisely located between the bladder musculature and its adherent fatty tissue with peritoneum. The inferior limit of dissection is the cervix in the female or the seminal vesicles in the male.
If there is tumor invading the seminal vesicles posteriorly and indenting the base of the bladder anteriorly, resection of the seminal vesicles and a part of the prostate may be necessary.
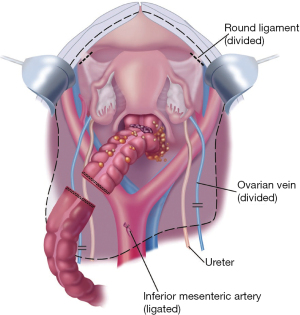
The peritoneal incision around the pelvis is connected to the peritoneal incisions of the right and left paracolic sulci (Figure 9). In the female, the round ligaments are divided as they enter the internal inguinal ring. The right and left ureters are identified and preserved. In women, the right and left ovarian veins are ligated at the level of the lower pole of the kidney and divided. A linear stapler is used to divide the sigmoid colon just above the limits of the pelvic tumor. The vascular supply of the distal portion of the rectosigmoid colon is traced back to its origin on the aorta. The inferior mesenteric artery is ligated, suture-ligated, and divided, which allows one to pack all of the viscera, including the proximal sigmoid colon, into the upper abdomen.
Electrosurgery is used to dissect at the limits of the mesorectum. The surgeon works in a centripetal fashion. Extraperitoneal ligation of the uterine arteries is performed just above the ureter and near the base of the bladder. The bladder is dissected away from the cervix, and the vagina is entered. The vaginal cuff anterior and posterior to the cervix is transected using electrosurgery, and the rectovaginal septum is exposed. The perirectal fat is divided beneath the peritoneal reflection so that all tumor that occupies the cul-de-sac is removed intact with the specimen. The rectal musculature is skeletonized using electrosurgery so that a stapler can be used to close the rectal stump.
Regional chemotherapy
The second part of the ultraradical local-regional strategy is intraperitoneal chemotherapy administered after a complete or near complete CRS. In retrospect, it is surprising that CRS alone has never been compared to CRS plus HIPEC. There seemed to be such a remarkable improvement in the outcome of surgery for MPM when HIPEC was added in phase II trials that the phase III trial never occurred. Standard of care was generally accepted around the globe as CRS plus HIPEC with a cisplatin-based regimen (26). How much of the markedly improved outcome was due to the new surgical techniques which included peritonectomy, visceral resections and a goal of complete visible removal of tumor and how much improvement was due to HIPEC will probably never be determined. A randomized trial of CRS ± HIPEC is likely to be considered unethical at this point in time.
The HIPEC treatment is given in the operating theater immediately after the complete CRS has been accomplished. It should be given prior to the performance of intestinal anastomoses and closure of the abdominal incision. This timely application of heated chemotherapy to all abdominal and pelvic surfaces including those that will be involved in suture lines avoids tumor cell entrapment (27).Also, an open HIPEC methodology is recommended in order to manually distribute the warm chemotherapy solution to abdominal and pelvic surfaces that are adherent to one another. Small bowel loops and the deep recesses between the leaves of the small bowel mesentery are at high risk for little or no access to the HIPEC (28).
The tubes for warm chemotherapy solution inflow and then drainage are shown in Figure 10. The skin edges are elevated on a Thompson self-retaining retractor (Thompson Surgical Instruments, Traverse City, MI, USA) (Figure 11).The HIPEC is usually continued for 90 minutes. The chemotherapy regimen is bidirectional with intravenous and intraperitoneal drugs that are augmented in their cytotoxicity by heat. The standardized HIPEC orders are shown in Table 3.

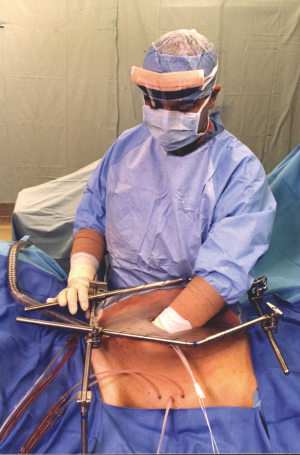
Table 3
| Intraperitoneal chemotherapy |
| (I) Add cisplatin to 3 L 1.5% dextrose peritoneal dialysis solution |
| (II) Add doxorubicin to the same 3 L 1.5% peritoneal dialysis solution |
| (III) Dose of cisplatin is 50 mg/m2 and doxorubicin is 15 mg/m2 for 90-minute HIPEC treatment |
| Intravenous chemotherapy |
| (I) Add ifosfamide 1,300 mg/m2 to 1 L 0.9% sodium chloride. Begin continuous IV infusion over 90 minutes simultaneous with intraperitoneal chemotherapy |
| (II) Add mesna disulfide 260 mg/m2 in 100 mL 0.9% sodium chloride to be given IV as a bolus 15 minutes prior to ifosfamide infusion |
| (III) Add mesna disulfide 260 mg/m2 in 100 mL 0.9% sodium chloride to be given IV as a bolus 4 hours after ifosfamide infusion |
| (IV) Add mesna disulfide 260 mg/m2 in 100 mL 0.9% sodium chloride to be given IV as a bolus 8 hours after ifosfamide infusion |
However, additions to the HIPEC regimen used in MPM have been studied and further improvements in outcome documented. Sugarbaker and Chang reported in a retrospective analysis of 129 patients with MPM who had CRS plus HIPEC (14). Forty-two patients had CRS and HIPEC with a 5-year survival of 44%. If the perioperative chemotherapy was HIPEC plus EPIC paclitaxel, the 5-year survival was 52%. If ultraradical intraperitoneal chemotherapy with HIPEC, EPIC and NIPEC was used, the 5-year survival increased to 75% (P=0.0374). With these additional patients treated with HIPEC, EPIC and NIPEC a propensity matched analysis was performed. The survival of the HIPEC, EPIC and NIPEC group was significantly improved when compared to HIPEC and EPIC (P=0.0263) (14).
The standardized orders for EPIC and NIPEC paclitaxel is shown in Table 4. EPIC paclitaxel is given on postoperative days 1–5. The NIPEC paclitaxel is given through an intraperitoneal port for 5 additional 5-day treatments every 4 weeks. The daily dose of intraperitoneal paclitaxel can be escalated as tolerated from 20 to 40 mg/m2.Usually, the maximum tolerated dose is 30 mg/m2 for 5 days in a row.
Table 4
| (I) Paclitaxel _________ mg (20 to 40 mg/m2 × _________ m2) (maximum dose =80 mg) in 1,000 mL 6% Hespan® (B. Braun, Irvine, CA) via Tenckhoff catheter daily: start date _________, stop date _________ |
| (II) Instill as rapidly as possible via Tenckhoff catheter. Dwell for 23 hours. Drain from Jackson-Pratt drains for one hour prior to next instillation |
| (III) During the initial 6 hours after chemotherapy infusion, the patient’s bed should be kept flat. The patient should be on the right-side during instillation. Turn at 30 minutes post instillation onto the left side and continue to change sides at 30-minute intervals for 6 hours |
| (IV) Monitor with pulse oximeter during the first 6 hours of intraperitoneal chemotherapy |
| (V) Continue to drain abdominal cavity by Jackson-Pratt drains after the last dose of intraperitoneal chemotherapy |
There are several unique features of the NIPEC paclitaxel that should be noted. First, the drug is given as a series of instillations over 5 days. The chemotherapy solution of paclitaxel in a starch carrier is instilled and dwells for 24 hours before the next instillation. The starch solution is used rather than an aqueous solution in order to minimize the progression of an adhesive process (HESPAN, 6% hydroxyethyl starch, B. Braun Medical, Melsungen, Germany). It also supplements the time for contact of the chemotherapy solution with the abdominal and pelvic surfaces (29).
Second, the 5 daily instillations have the capability to interact with a tumor nodule layer by layer over the 5 days of treatment. This “peel the onion” phenomenon requires repeated doses of paclitaxel (30).
The surgical placement of an intraperitoneal port that provides complication-free access to the peritoneal space is an essential part of this ultraradical local-regional strategy. If the port insertion is performed by inexperienced physicians, up to 50% of patients will have an interruption of treatment (31). A port inserted at the completion of CRS and used for the instillation of EPIC paclitaxel is recommended (32).
Finally, although greatly reduced in number, MPM patients following treatment with the ultraradical local-regional regimen may recur. Careful follow-up with CT of chest, abdomen and pelvis is recommended. If by CT the recurrence appears to be resectable an iterative cytoreduction is indicated (33,34). HIPEC should be used again in a reoperative setting if CRS is complete or near complete. Additional combined intraperitoneal pemetrexed and systemic cisplatin is an excellent choice for additional chemotherapy if the patient can tolerate further treatment (Table 5).
Table 5
| (I) Folic acid 1 mg daily p.o. 1 to 3 weeks before the first dose of pemetrexed and continue for 1 to 3 weeks after discontinuation of pemetrexed |
| (II) Vitamin B12 (Cyanocobalamin) 1,000 μg intramuscularly 1 to 3 weeks before the first dose of pemetrexed and repeat every 9 weeks until discontinuation of pemetrexed |
| (III) Start peripheral IV with large-bore catheter 2 hours prior to chemotherapy administration |
| (IV) Run D5½ normal saline at 200 mL/hour |
| (V) Pemetrexed (500 mg/m2) _________ mg in 1,000 mL 1.5% dextrose peritoneal dialysis solution as a 60-minute rapid infusion through intraperitoneal port |
| (VI) At 30 minutes after pemetrexed infusion, give 12.5 g mannitol in 100 mL normal saline over 15 minutes intravenously |
| (VII) At 60 minutes after pemetrexed infusion, give cisplatin (75 mg/m2) _________ mg in 250 mL normal saline over 120 minutes intravenously |
In summary, an ultraradical local-regional strategy is recommended for the management of this disease that progresses within the peritoneal spaces. The strategy begins with proper patient selection. The histology should be epithelial, not sarcomatoid or biphasic. The Ki67 proliferation index performed on the biopsy for diagnosis should be <7%. The CCTF should be limited, preferably absent. Although young age is not a requirement, fitness for surgery and the subsequent surgery is necessary. Extensive prior surgery decreased favorable outcome and increases operative morbidity. Neoadjuvant chemotherapy should be avoided. The treatment begins with a major surgical intervention that can require 10–13 hours. Up to 6 peritonectomy procedures and numerous visceral resections are performed prior to HIPEC. All of the cytoreduction should be performed at a single surgical procedure. The EPIC and NIPEC with paclitaxel are continued for 6 months or whatever time is necessary to complete 5 week-long cycles of NIPEC paclitaxel. Late recurrence, even a decade after instillation of treatment, may occur so that long-term follow-up is indicated.
Acknowledgments
The authors appreciate the Editor for providing the opportunity to submit this review article.
Funding: None.
Footnote
Reporting Checklist: The author has completed the Narrative Review Reporting Checklist. Available at: http://dx.doi.org/10.21037/pcm-21-2
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pcm-21-2). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sugarbaker PH, Welch LS, Mohamed F, et al. A review of peritoneal mesothelioma at the Washington Cancer Institute. Surg Oncol Clin N Am 2003;12:605-21. [Crossref] [PubMed]
- Sugarbaker PH, Chang D. Cytoreductive surgery plus HIPEC with and without NIPEC for malignant peritoneal mesothelioma: A propensity score analysis. Ann Surg Oncol 2021; Epub ahead of print. [Crossref] [PubMed]
- Baas P, Scherpereel A, Nowak AK, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet 2021;397:375-86. [Crossref] [PubMed]
- Yan TD, Brun EA, Cerruto CA, et al. Prognostic indicators for patients undergoing cytoreductive surgery and perioperative intraperitoneal chemotherapy for diffuse malignant peritoneal mesothelioma. Ann Surg Oncol 2007;14:41-9. [Crossref] [PubMed]
- Kepenekian V, Elias D, Passot G, et al. Diffuse malignant peritoneal mesothelioma: Evaluation of systemic chemotherapy with comprehensive treatment through RENAPE Database Multi-Institutional Retrospective Study. Eur J Cancer 2016;65:69-79. [Crossref] [PubMed]
- Garcia-Carbonero R, Paz-Ares L. Systemic chemotherapy in the management of malignant peritoneal mesothelioma. Eur J Surg Oncol 2006;32:676-81. [Crossref] [PubMed]
- Yan TD, Popa E, Brun EA, et al. Sex difference in diffuse malignant peritoneal mesothelioma. Br J Surg 2006;93:1536-42. [Crossref] [PubMed]
- Cerruto CA, Brun EA, Chang D, et al. Prognostic significance of histomorphologic parameters in diffuse malignant peritoneal mesothelioma. Arch Pathol Lab Med 2006;130:1654-61. [Crossref] [PubMed]
- Kusamura S, Torres Mesa PA, Cabras A, et al. The role of Ki-67 and pre-cytoreduction parameters in selecting diffuse malignant peritoneal mesothelioma (DMPM) patients for cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). Ann Surg Oncol 2016;23:1468-73. [Crossref] [PubMed]
- Gilani SNS, Mehta A, Garcia-Fadrique A, et al. Outcomes of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal mesothelioma and predictors of survival. Int J Hyperthermia 2018;34:578-84. [Crossref] [PubMed]
- Jacquet P, Stephens AD, Averbach AM, et al. Analysis of morbidity and mortality in 60 patients with peritoneal carcinomatosis treated by cytoreductive surgery and heated intraoperative intraperitoneal chemotherapy. Cancer 1996;77:2622-9. [Crossref] [PubMed]
- Yan TD, Welch L, Black D, et al. A systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for diffuse malignant peritoneal mesothelioma. Ann Oncol 2007;18:827-34. [Crossref] [PubMed]
- Sugarbaker PH, Chang D, Jelinek JS. In 100 patients with malignant peritoneal mesothelioma, concerning CT features predicted outcome of treatment. Eur J Surg Oncol 2021; Epub ahead of print. [Crossref] [PubMed]
- Sugarbaker PH, Chang D. Long-term regional chemotherapy for patients with epithelial malignant peritoneal mesothelioma results in improved survival. Eur J Surg Oncol 2017;43:1228-35. [Crossref] [PubMed]
- Simkens GA, van Oudheusen TR, Luyer MD, et al. Predictors of severe morbidity after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for patients with colorectal peritoneal carcinomatosis. Ann Surg Oncol 2016;23:833-41. [Crossref] [PubMed]
- Bekhor E, Carr J, Hofstedt M, et al. The safety of iterative cytoreductive surgery and HIPEC for peritoneal carcinomatosis: A high volume center prospectively maintained database analysis. Ann Surg Oncol 2020;27:1448-55. [Crossref] [PubMed]
- Le Roy F, Gelli M, Hollebecque A, et al. Conversion to complete cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma after bidirectional chemotherapy. Ann Surg Oncol 2017;24:3640-6. [Crossref] [PubMed]
- Ishigami H, Fujiwara Y, Fukushima R, et al. Phase III trial comparing intraperitoneal and intravenous paclitaxel plus S-1 versus cisplatin plus S-1 in patients with gastric cancer with peritoneal metastasis: PHOENIX-GC Trial. J Clin Oncol 2018;36:1922-9. [Crossref] [PubMed]
- Jónsdóttir B, Lomnytska M, Poromaa IS, et al. The peritoneal cancer index is a strong predictor of incomplete cytoreductive surgery in ovarian cancer. Ann Surg Oncol 2021;28:244-51. [Crossref] [PubMed]
- da Silva RG, Sugarbaker PH. Analysis of prognostic factors in seventy patients having a complete cytoreduction plus perioperative intraperitoneal chemotherapy for carcinomatosis from colorectal cancer. J Am Coll Surg 2006;203:878-86. [Crossref] [PubMed]
- Van der Speeten K, Stuart OA, Sugarbaker PH. Cancer chemotherapy for peritoneal metastases: Pharmacology and treatment. In: Sugarbaker PH. Editor. Cytoreductive Surgery & Perioperative Chemotherapy for Peritoneal Surface Malignancy. Textbook and Video Atlas. 2nd Edition. Woodbury, CT: Cine-Med Publishing, 2017:47-82.
- Sugarbaker PH, van der Speeten K. An overview of peritonectomy, visceral resection, and therapeutic laparoscopy for peritoneal surface malignancy. In: Sugarbaker PH. Editor. Cytoreductive Surgery & Perioperative Chemotherapy for Peritoneal Surface Malignancy. Textbook and Video Atlas. 2nd Edition. Woodbury, CT: Cine-Med Publishing, 2017:17-46.
- Sugarbaker PH. Circumferential cutaneous traction for exposure of the layers of the abdominal wall. J Surg Oncol 2008;98:472-5. [Crossref] [PubMed]
- Sugarbaker PH. Dissection by electrocautery with a ball tip. J Surg Oncol 1994;56:246-8. [Crossref] [PubMed]
- Deraco M, Baratti D, Kusamura S, et al. Surgical technique of parietal and visceral peritonectomy for peritoneal surface malignancies. J Surg Oncol 2009;100:321-8. [Crossref] [PubMed]
- Sugarbaker PH, Turaga KK, Alexander HR Jr, et al. Management of malignant peritoneal mesothelioma using cytoreductive surgery and perioperative chemotherapy. J Oncol Pract 2016;12:928-35. [Crossref] [PubMed]
- Sethna KS, Sugarbaker PH. New prospects for the control of peritoneal surface dissemination of gastric cancer using perioperative intraperitoneal chemotherapy. Cancer Therapy 2004;2:79-84.
- Sugarbaker PH, Averbach AM, Jacquet P, et al. A simplified approach to hyperthermic intraoperative intraperitoneal chemotherapy (HIIC) using a self retaining retractor. In: Sugarbaker PH. Editor. Peritoneal Carcinomatosis: Principles of Management. Boston: Kluwer, 1996:415-21.
- Mohamed F, Marchettini P, Stuart OA, et al. A comparison of hetastarch and peritoneal dialysis solution for intraperitoneal chemotherapy delivery. Eur J Surg Oncol 2003;29:261-5. [Crossref] [PubMed]
- Kuh HJ, Jang SH, Wientjes MG, et al. Determinants of paclitaxel penetration and accumulation in human solid tumor. J Pharmacol Exp Ther 1999;290:871-80. [PubMed]
- Walker JL, Armstrong DK, Huang HQ, et al. Intraperitoneal catheter outcomes in a phase III trial of intravenous versus intraperitoneal chemotherapy in optimal stage III ovarian and primary peritoneal cancer: a Gynecologic Oncology Group study. Gynecol Oncol 2006;100:27-32. [Crossref] [PubMed]
- Sugarbaker PH, Bijelic L. Adjuvant bidirectional chemotherapy using an intraperitoneal port. Gastroenterol Res Pract 2012;2012:752643. [Crossref] [PubMed]
- Ihemelandu C, Bijelic L, Sugarbaker PH. Iterative cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for recurrent or progressive diffuse malignant peritoneal metastases: Clinicopathologic characteristics and survival outcome. Ann Surg Oncol 2015;22:1680-5. [Crossref] [PubMed]
- Llanos MD, Sugarbaker PH. Symptoms, signs and radiologic findings in patients having reoperative surgery for malignant peritoneal mesothelioma. Eur J Surg Oncol 2017;43:138-43. [Crossref] [PubMed]
Cite this article as: Sugarbaker PH. Malignant peritoneal mesothelioma: a narrative review of the rationale for and results of treatment using ultraradical local-regional strategy. Precis Cancer Med 2021;4:21.

