
Management of acquired resistance to ALK inhibitors: repeat biopsy to characterize mechanisms of resistance significantly impacts clinical outcomes
Introduction
The discovery of anaplastic lymphoma kinase (ALK) rearrangements in patients with non-small cell lung cancer (NSCLC) in 2007 and the subsequent development of ALK tyrosine kinase inhibitors (TKI) paved the way for precision oncology in the treatment of NSCLC (1). Crizotinib, a multi-kinase inhibitor with activity against ALK and mesenchymal epithelial transition (MET) receptors, was the first TKI to be approved by the US Food and Drug Administration (FDA) for treatment of ALK-rearranged (ALK+) NSCLC, based on PROFILE1007 and 1014 trials (2,3). The second generation ALK TKIs, ceritinib, alectinib and brigatinib are more selective and potent when compared to crizotinib. Alectinib and brigatinib have demonstrated superior efficacy in patients with ALK+ NSCLC when compared to crizotinib in treatment naïve patients (4-6). Both are now FDA approved for use in treatment naïve patients with ALK+ NSCLC. Ceritinib is also FDA approved in the first line setting based on superior progression free survival (PFS) when compared to platinum doublet chemotherapy (7). Recently, lorlatinib, a third generation ALK inhibitor showed improved PFS compared to crizotinib in treatment naïve patients (8). Moreover, next generation ALK TKIs have superior intracranial efficacy compared to crizotinib. Despite a growing portfolio of selective ALK TKIs, resistance mechanisms inevitably develop and challenges in management of disease progression remain. Herein we discuss the crucial role repeat tumor biopsy has in impacting the clinical outcomes of patients with ALK+ NSCLC.
ALK resistance mechanisms and management
ALK-dependent resistance mechanisms
Secondary ALK resistance mutations are less common with crizotinib (20–30%) than with second-generation TKIs (56%) (9,10). In addition to being significantly less potent than next generation ALK TKIs, crizotinib has poor central nervous system (CNS) penetration (11,12). More potent and highly selective second generation ALK TKIs not only inhibit common crizotinib resistance mutations (13-17), but also have superior central CNS efficacy (5,6,8). The two most common secondary ALK resistance mutations to crizotinib, L1196M and G1269A remain sensitive to all next generation ALK TKIs (13,14,16,17). Sixty nine percent of patients with disease progression on crizotinib have wild type ALK (10). Therefore, crizotinib resistance is less frequently due to ALK dependent resistance mechanisms and more likely due to inadequate potency and CNS activity of crizotinib. With the exception of G1202R, which is seen in 2% of patients with crizotinib resistance (9,10,18), all next generation ALK TKIs remain effective for patients with disease progression on crizotinib. Treatment with a next-generation ALK TKI in the second line setting yields a median PFS of 6.9 to 11.1 months depending upon the TKI used (19-22). Hence, repeat biopsy at disease progression may have limited value in crizotinib-resistant disease.
The second generation ALK TKI, alectinib has increasingly been adopted as the first line of therapy for metastatic ALK+ NSCLC. The most common ALK dependent resistance mechanism with alectinib is the G1202R solvent front mutation, found in 29% of patients (10). Other ALK dependent resistance mechanisms include I1171T/S, and V1180L mutations in smaller proportions (12% and 6% respectively) (10). Ceritinib and brigatinib have been shown to be active against I1171T/S and V1180L mutations in preclinical studies and clinical reports (23,24). In a phase II trial of ceritinib for patients with alectinib resistant tumors, ORR was 25% and mPFS was only 3.7 months (95% CI: 1.9–5.3 months) (25). This study did not characterize post-alectinib resistance mechanisms and their correlation with clinical outcome on ceritinib. Another phase II trial of brigatinib in patients with disease progression on alectinib showed an ORR of 17% and a mPFS of only 4.4 months (95% CI: 1.8–5.6 months) (26). In this trial, 9 patients had a repeat biopsy at disease progression on alectinib, of which two had I1171N, one had I1171T, two had V1180L and one had G1202R mutations. While patients with I1171N/T and V1180L mutations had either a partial response or stable disease, the patient with G1202R mutation had disease progression as their best response to ceritinib. Both these trials were not biomarker driven. But in the latter trial, the benefit of repeat biopsy to identify resistance mechanisms in the post-alectinib setting is obvious.
The G1202R mutation is the most common resistance mutation in response to not only alectinib but to all second generation ALK TKIs (9,10,18). Lorlatinib is a third generation, ATP-competitive, reversible ALK and ROS1 TKI designed specifically to overcome known ALK-dependent resistance mechanisms. Lorlatinib is active against most secondary ALK resistance mutations that develop with second generation ALK TKIs and is the only FDA approved ALK TKI active against G1202R mutation (8,21). Despite lorlatinib’s broad activity against ALK aberrations, in phase I and II studies, patients with second-generation TKI-resistance had a lower objective response rate (ORR) compared to those with crizotinib resistance (39% vs. 69%) (Table 1) (27). As expected, analysis of tumor tissue and ctDNA from patients enrolled in the phase II trial of lorlatinib showed that G1269A, F1174X, and L1196M mutations were more common in crizotinib treated patients compared to G1202R/del, detected in up to 55% of patients who had been treated with one or more second generation ALK TKIs (21). Lorlatinib had an ORR of 42–89% among patients with the five most common ALK mutations observed in patients who had received prior ALK TKIs. In patients who had received prior crizotinib, ORR was similar among those with and without secondary ALK resistance mutation detected by tissue genotyping (73% and 74% respectively). However, among patients who had received prior second generation ALK TKIs, the ORR was higher in patients with secondary ALK mutations compared to those without an ALK mutation detected by tissue genotyping (69% vs. 27%). About one-third of patients with detectable ALK mutations had more than one ALK mutation, possibly indicative of compound ALK mutations. These patients with compound ALK mutations had a worse ORR than those with a single ALK mutation. Therefore, particularly in patients who have disease progression on second generation ALK TKIs, presence of secondary ALK resistance mutation is an important predictor of response to lorlatinib.
Table 1
| ALK mutation status* | Objective response rate to lorlatinib | Median progression free survival with lorlatinib (months) (95% CI) | |||
|---|---|---|---|---|---|
| Prior crizotinib | Prior second generation ALK TKI | Prior crizotinib | Prior second generation ALK TKI | ||
| Any | 69.5% | 38.7% | 11.1 (8.2–NR) | 6.9 (5.4–8.2) | |
| ALK mutation present | 74% | 69% | NR (2.6–NR) | 11 (6.9–NR) | |
| ALK mutation absent | 73% | 27% | 12.5 (6.9–NR) | 5.4 (3.9–6.9) | |
| One ALK mutation | – | 75% | – | – | |
| More than one ALK mutation | – | 56% | – | – | |
*, by tissue genotyping. ALK, anaplastic lymphoma kinase; TKI, tyrosine kinase inhibitor; NR, not reached.
Resistance to lorlatinib itself, eventually develops with, often, diverse mechanisms including compound ALK resistance mutations (28). In a patient who developed disease progression on lorlatinib, compound ALK L1198F and C1156Y mutations re-sensitized the tumor to crizotinib (29), once again highlighting the importance of repeat biopsy to ascertain resistance mechanisms. Additionally, analysis of repeat biopsies from twenty patients with lorlatinib resistance, 19 of whom had received two or more prior ALK inhibitors, showed stepwise development of compound ALK mutations conferring high-level lorlatinib resistance (30). This indicates that sequential use of ALK TKIs could select for compound ALK dependent resistance mutations to lorlatinib. Therefore, repeat biopsy at disease progression after crizotinib or second generation ALK TKIs may be valuable in strategic sequencing of TKIs in order to minimize the risk of selecting for lorlatinib-resistant compound mutations.
ALK independent resistance mechanisms
In about three quarters of patients who receive ALK TKIs, no ALK mutation is detected on tissue genotyping at the time of progression, indicating the presence of ALK-independent resistance mechanisms (10). Major ALK-independent resistance mechanisms include activation of bypass signaling pathways and phenotypic transformation. Activation of EGFR (epidermal growth factor receptor) and other members of HER (human epidermal growth factor receptor) receptor family has been implicated in ALK TKI resistance (9,31). However, the safety and efficacy of dual EGFR or HER2 and ALK blockade is unknown. In a patient with alectinib resistance without ALK or EGFR aberrations, MET amplification was detected by FISH (32). This patient responded to crizotinib which is also a potent MET TKI. Additionally, phenotypic changes such as epithelial-to-mesenchymal transition (EMT) and transformation to small cell lung cancer can lead to ALK TKI resistance (10). Patients with ALK-independent resistance mechanisms are less likely to respond to lorlatinib and alternative combinatorial treatment strategies may be required. However, combination regimen should be attempted on clinical trials due to increased risk of toxicities. If enrolment in a clinical trial is not feasible, patients may still derive benefit from platinum/pemetrexed with ALK TKI or localized therapy in case of oligoprogression (Figure 1) (33,34).
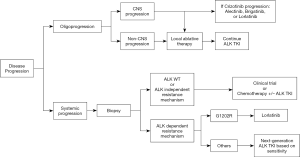
Method of ALK mutation detection
Repeat biopsy at disease progression may not always be safe or feasible. Although plasma genotyping using ctDNA seems like an attractive alternative to tumor re-biopsy at disease progression, plasma ctDNA testing has a sensitivity of 61% and a significant false-negative rate of 39% (35). Among patients who were treated with lorlatinib following resistance to one or more second generation ALK TKIs, no difference in PFS was observed between those with and without ALK mutations detected by plasma ctDNA. But in the same group of patients, ALK mutation detected by tissue genotyping was associated with significantly longer PFS compared to those without an ALK mutation. Plasma ctDNA testing at disease progression is currently of limited utility in choice of subsequent therapy due to significant false negative rate and therefore, every effort to re-biopsy the tumor should be made at disease progression as long as it is safe and feasible.
Conclusions
The success of precision oncology lies in our ability to individualize treatments based on the genomic characteristics of the tumor. Secondary ALK resistance mechanisms are varied and TKI dependent. Lorlatinib, is efficacious in patients with disease progression on crizotinib and second generation ALK TKIs. However, ALK-dependent resistance mechanisms detected by tissue genotyping appear to be predictive of lorlatinib efficacy. Additionally, tumors with compound ALK-dependent resistance mechanisms appear to respond poorly to lorlatinib. Non-biomarker driven use of lorlatinib at disease progression is antithetical to the premise of precision oncology, in that it may not benefit all patients with disease progression on older ALK TKIs, while imposing a significant economic burden. Therefore, repeat tumor biopsy at disease progression in order to determine resistance mechanisms is consequential to clinical outcomes of patients with ALK+ NSCLC.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor Grace K. Dy for the series “Evidence and Controversies in the treatment of metastatic NSCLC” published in Precision Cancer Medicine. The article has undergone peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pcm-21-5). The series “Evidence and Controversies in the treatment of metastatic NSCLC” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethics Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [Crossref] [PubMed]
- Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol 2016;17:234-42. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non–Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Camidge DR, Kim HR, Ahn M-J, et al. Brigatinib versus Crizotinib in ALK-Positive Non–Small-Cell Lung Cancer. N Engl J Med 2018;379:2027-39. [Crossref] [PubMed]
- Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389:917-29. [Crossref] [PubMed]
- Shaw AT, Bauer TM, de Marinis F, et al. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N Engl J Med 2020;383:2018-29. [Crossref] [PubMed]
- Katayama R, Shaw AT, Khan TM, et al. Mechanisms of Acquired Crizotinib Resistance in ALK-Rearranged Lung Cancers. Sci Transl Med 2012;4:120ra17. [Crossref] [PubMed]
- Gainor JF, Dardaei L, Yoda S, et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov 2016;6:1118-33. [Crossref] [PubMed]
- Costa DB, Kobayashi S, Pandya SS, et al. CSF Concentration of the Anaplastic Lymphoma Kinase Inhibitor Crizotinib. J Clin Oncol 2011;29:e443-5. [Crossref] [PubMed]
- Rangachari D, Yamaguchi N, VanderLaan PA, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer 2015;88:108-11. [Crossref] [PubMed]
- Kodama T, Tsukaguchi T, Yoshida M, et al. Selective ALK inhibitor alectinib with potent antitumor activity in models of crizotinib resistance. Cancer Lett 2014;351:215-21. [Crossref] [PubMed]
- Sakamoto H, Tsukaguchi T, Hiroshima S, et al. CH5424802, a Selective ALK Inhibitor Capable of Blocking the Resistant Gatekeeper Mutant. Cancer Cell 2011;19:679-90. [Crossref] [PubMed]
- Friboulet L, Li N, Katayama R, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov 2014;4:662-73. [Crossref] [PubMed]
- Zhang S, Anjum R, Squillace R, et al. The Potent ALK Inhibitor Brigatinib (AP26113) Overcomes Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in Preclinical Models. Clin Cancer Res 2016;22:5527-38. [Crossref] [PubMed]
- Zou HY, Friboulet L, Kodack DP, et al. PF-06463922, an ALK/ROS1 Inhibitor, Overcomes Resistance to First and Second Generation ALK Inhibitors in Preclinical Models. Cancer Cell 2015;28:70-81. [Crossref] [PubMed]
- Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of Resistance to Crizotinib in Patients with ALK Gene Rearranged Non–Small Cell Lung Cancer. Clin Cancer Res 2012;18:1472-82. [Crossref] [PubMed]
- Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-Rearranged Non–Small-Cell Lung Cancer. N Engl J Med 2014;370:1189-97. [Crossref] [PubMed]
- Huber RM, Hansen KH, Paz-Ares Rodríguez L, et al. Brigatinib in Crizotinib-Refractory ALK+ NSCLC: 2-Year Follow-up on Systemic and Intracranial Outcomes in the Phase 2 ALTA Trial. J Thorac Oncol 2020;15:404-15. [Crossref] [PubMed]
- Shaw AT, Solomon BJ, Besse B, et al. ALK Resistance Mutations and Efficacy of Lorlatinib in Advanced Anaplastic Lymphoma Kinase-Positive Non–Small-Cell Lung Cancer. J Clin Oncol 2019;37:1370-9. [Crossref] [PubMed]
- Ou SI, Gadgeel SM, Barlesi F, et al. Pooled overall survival and safety data from the pivotal phase II studies (NP28673 and NP28761) of alectinib in ALK-positive non-small-cell lung cancer. Lung Cancer 2020;139:22-7. [Crossref] [PubMed]
- Sehgal K, Peters MLB, VanderLaan PA, et al. Activity of Brigatinib in the Setting of Alectinib Resistance Mediated by ALK I1171S in ALK-Rearranged Lung Cancer. J Thorac Oncol 2019;14:e1-3. [Crossref] [PubMed]
- Katayama R, Friboulet L, Koike S, et al. Two Novel ALK Mutations Mediate Acquired Resistance to the Next-Generation ALK Inhibitor Alectinib. Clin Cancer Res 2014;20:5686-96. [Crossref] [PubMed]
- Hida T, Seto T, Horinouchi H, et al. Phase II study of ceritinib in alectinib-pretreated patients with anaplastic lymphoma kinase-rearranged metastatic non-small-cell lung cancer in Japan: ASCEND-9. Cancer Sci 2018;109:2863-72. [Crossref] [PubMed]
- Lin JJ, Zhu VW, Schoenfeld AJ, et al. Brigatinib in Patients With Alectinib-Refractory ALK-Positive NSCLC. J Thorac Oncol 2018;13:1530-8. [Crossref] [PubMed]
- Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol 2018;19:1654-67. [Crossref] [PubMed]
- Zhu VW, Nagasaka M, Madison R, et al. A Novel Sequentially Evolved EML4-ALK Variant 3 G1202R/S1206Y Double Mutation In Cis Confers Resistance to Lorlatinib: A Brief Report and Literature Review. JTO Clin Res Rep 2021;2:100116.
- Shaw AT, Friboulet L, Leshchiner I, et al. Resensitization to Crizotinib by the Lorlatinib ALK Resistance Mutation L1198F. N Engl J Med 2016;374:54-61. [Crossref] [PubMed]
- Yoda S, Lin JJ, Lawrence MS, et al. Sequential ALK Inhibitors Can Select for Lorlatinib-Resistant Compound ALK Mutations in ALK-Positive Lung Cancer. Cancer Discov 2018;8:714-29. [Crossref] [PubMed]
- Tanizaki J, Okamoto I, Okabe T, et al. Activation of HER Family Signaling as a Mechanism of Acquired Resistance to ALK Inhibitors in EML4-ALK–Positive Non–Small Cell Lung Cancer. Clin Cancer Res 2012;18:6219-26. [Crossref] [PubMed]
- Gouji T, Takashi S, Mitsuhiro T, et al. Crizotinib Can Overcome Acquired Resistance to CH5424802: Is Amplification of the MET Gene a Key Factor? J Thorac Oncol 2014;9:e27-8. [Crossref] [PubMed]
- Lin JJ, Schoenfeld AJ, Zhu VW, et al. Efficacy of Platinum/Pemetrexed Combination Chemotherapy in ALK-Positive NSCLC Refractory to Second-Generation ALK Inhibitors. J Thorac Oncol 2020;15:258-65. [Crossref] [PubMed]
- Weickhardt AJ, Scheier B, Burke JM, et al. Local Ablative Therapy of Oligoprogressive Disease Prolongs Disease Control by Tyrosine Kinase Inhibitors in Oncogene-Addicted Non–Small-Cell Lung Cancer. J Thorac Oncol 2012;7:1807-14. [Crossref] [PubMed]
- Shaw AT, Solomon BJ, Besse B, et al. ALK Resistance Mutations and Efficacy of Lorlatinib in Advanced Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2019;37:1370-9. [Crossref] [PubMed]
Cite this article as: Hegde A, Khalil M. Management of acquired resistance to ALK inhibitors: repeat biopsy to characterize mechanisms of resistance significantly impacts clinical outcomes. Precis Cancer Med 2021;4:20.

