The use of stereotactic body radiotherapy in pulmonary carcinoid tumors: a case series
Introduction
Pulmonary carcinoid (PC) tumors are rare neoplasms accounting for roughly 2% of invasive lung cancers (1,2). These tumors have an annual incidence of 2 per 100,000 persons (1). PCs arise from neuroendocrine cells with the majority being well differentiated, low grade typical carcinoid (TC) tumors with <2 mitoses per 10 high power fields (hpfs) without necrosis (2). A minority of tumors may present as more aggressive neoplasms with 2–10 mitoses per 10 hpfs and may demonstrate necrosis; these tumors are categorized as intermediate grade, atypical carcinoid (AC) tumors (2), The majority of PCs are sporadic; however, nearly 5% are associated with multiple endocrine neoplasm type 1 (MEN-1) (3,4).
The majority of PCs are localized, with approximately 15% of patients having lymph node involvement and 3% having distant metastatic disease at time of diagnosis (5). Although surgical resection is standard treatment for PCs, not all patients are surgical candidates due to underlying medical co-morbidities, metastatic disease, or patient preference.
Traditionally, these tumors have been classified as radiation resistant and thus radiation has not been a typical treatment regimen for PCs (6). Stereotactic body radiotherapy (SBRT) is an effective way to deliver curative intent radiation to tumors, commonly inoperable non-small cell lung cancer (NSCLC) (7,8). Additionally, radiation may be considered for local control for enlarging primary tumors in the setting of stable distant disease.
There is a paucity of data examining SBRT in PCs. The literature contains a few case series and one larger retrospective analysis; however, these reports focus on early-stage disease with advanced stage and metastatic disease being exclusion criteria (6,9,10).
As such, we aimed to review the outcomes of patients at our institution who were ineligible for surgery with a diagnosis of PCs who received SBRT for definitive treatment in early-stage disease or for local control in the setting of advanced or metastatic disease. We present the following article in accordance with the AME Case Series reporting checklist (available at http://dx.doi.org/10.21037/pcm-20-71).
Methods
Data collection
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study received approval from Louisiana State University Health Sciences Center-New Orleans and Ochsner Clinic Foundation’s Institutional Review Boards (IRB# 987; 2011.038.C). Individual consent for this retrospective analysis was waived. All patients over the age of 18 years who were evaluated by the New Orleans Louisiana Neuroendocrine Tumor Specialists (NOLANETS) team with a pathological confirmed diagnosis of PC per the World Health Organization criteria and received SBRT from April 2013 to July 2020. Patients with a pathological diagnosis and who had pre and post treatment imaging were included. Patients were included in our study regardless of tumor stage, Eastern Cooperative Oncology Group (ECOG) performance status (PS), or previous systemic therapy utilization.
Each patient was presented at a multidisciplinary tumor board and their case reviewed by a thoracic surgeon, radiation oncologist, and medical oncologist. Patients were either deemed unfit for surgical resection or refused surgical intervention.
Patient demographics (sex, date of birth), prior treatments, ECOG PS, location of primary tumor and metastatic disease, pathologic characteristics (Ki-67 proliferative rate, typical vs. atypical PC), imaging results (CT, MRI), radiation treatment (radiation dose and fraction), and tumor measurements were recorded by medical chart review. Results are outlined in Table 1.
Table 1
| Patient | Age (years) | Sex | ECOG | Location of SBRT | TC/AC | KI67 (%) | Tumor size (mm) | T stage | Stage | Prior tx (1 = yes; 2 = no) | Dose, Gy/fraction, n | Follow-up (mo) | Decrease in tumor size (%) | Disease status1 | Status at last follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 65 | F | 0 | RUL; LUL | TC | NR | 8 | T1a | Stage IVA | 2 | 50/5 | 8.9 | 100 | CR | Alive |
| 2 | 53 | F | 0 | RUL | TC | 3 | 15 | T1b | Stage IA2 | 1 | 50/5 | 21.8 | 0 | SD | Alive |
| 3 | 78 | F | 1 | LUL | AC | 5 | 28 | T1c | Stage IVB | 1 | 50/5 | 8.9 | 31.1 | PR | Dead |
| 4 | 55 | F | 0 | LUL | TC | 10 | 7 | T1a | Stage IVB | 1 | 54/3 | 10.0 | 42.8 | PR | Alive |
| 5 | 72 | F | 0 | RUL | TC | 5 | 23 | T1c | Stage IVB | 1 | 42/3 | 14.0 | 39.1 | PR | Dead |
| 6 | 32 | F | 0 | L suprahilar LN | TC | 2.5 | 11 | T1a | Stage IIB | 1 | 60/8 | 15.9 | 41.5 | PR | Alive |
| 7 | 74 | F | 0 | RML | TC | 12 | 29 | T1c | Stage IVB | 1 | 50/5 | 25.7 | 37.9 | PR | Alive |
1, disease status by Response Evaluation Criteria in Solid Tumors (RECIST) criteria. AC, atypical carcinoid; CR, complete response; ECOG, Eastern Cooperative Oncology Group; LN, lymph node; LUL, left upper lobe; mo, months; NR, not recorded; RML, right middle lobe; RUL, right upper lobe; SBRT, stereotactic body radiotherapy; SD, stable disease; TC, typical carcinoid; tx, treatment.
Treatment planning
SBRT treatment planning and delivery was performed as follows: patients underwent CT simulation while immobilized in the BodyFix device (Elekta, Stockholm, Sweden). 4D-CT was obtained with patient free breathing using the Real-Time Position Management system (RPM, Varian Medical Systems, Palo Alto, CA, USA). Image data was then retrospectively sorted into 10 CT sets each corresponding to 10% of the respiratory cycle (GE LightSpeed RT scanner using Advantage 4D software, GE Healthcare, Chicago, IL, USA). A maximal intensity projection CT set was obtained from the 4D-CT. The internal target volume (ITV) was delineated by use of the maximum intensity projection (MIP) and 4D-CT sequences to define the PC target throughout all phases of the respiratory cycle. Treatment planning occurred on the average intensity projection CT set obtained from the 4D-CT. An isotropic margin of 3–5 mm from the ITV was applied to develop the planning target volume (PTV). Doses of 42–60 Gy in 3–8 fractions were prescribed to the 80% isodose line covering the PTV with at least 95% of the PTV receiving the prescribed dose. Dose fractionation was chosen based on location of PC and proximity to organs at risk. Treatment was delivered on a Varian TrueBeam linear accelerator with 6 MV photon beams optimized by volumetric modulated arc therapy and 1–3 non-coplanar beams. Daily cone-beam CT was performed prior to each SBRT fraction. All patients received follow up clinic appointments and imaging after radiation treatment.
Statistical analysis
Descriptive statistics were used to summarize patient characteristics, such as age, gender and other qualitative data. Response rate to therapy was assessed by CT as determined by the Response Evaluation Criteria in Solid Tumors (RECIST) criteria. The number of patients who had a complete response (CR), partial response (PR), stable disease (SD), or progression of disease (PD) was recorded.
Results
The demographics, tumor characteristics, and treatment course of our study population is outlined in Table 1. Seven patients with PC tumors who received SBRT between April 2013 to July 2020 were identified. Individual patient reasons for non-surgical approach are outlined in Table 2.
Table 2
| Patient | Rationale for SBRT |
|---|---|
| 1 | Two lung nodules |
| 2 | Morbid obesity, MEN1 |
| 3 | Metastatic with multiple treatment line failure |
| 4 | Non-surgical candidate due to previous lobectomy |
| 5 | Metastatic with localized therapy to metastatic site |
| 6 | Previous wedge resection |
| 7 | Non-surgical candidate due to tumor locality |
SBRT, stereotactic body radiotherapy; MEN-1, multiple endocrine neoplasm type 1.
All patients were female (100%). Median age at diagnosis was 65 years (range, 32–78 years). Six patients (85.7%) had an ECOG PS of 0, while 1 patient was an ECOG PS of 1 (14.3%).
A total of 8 PC tumors were treated in 7 patients, as one patient had two PC tumors. Six patients (85.7%) had TC histology, while 1 patient had an atypical PC (14.3%). One patient (14.3%) had multiple neuroendocrine neoplasia type 1. The Ki-67 proliferative rate was identified in 6 patients (85.7%). All patients had T1 disease; T1a, 3 (42.9%), T1b, 1 (14.3%), T1c 3 (42.9%). At the time of PC diagnosis, 1 patient was stage 1A2 (14.3%), 1 patient was stage IIB (14.3%), 1 patient was stage IVA (14.3%), and 4 patients were stage IVb (57.1%). Six patients (85.7%) had a lung primary and 1 patient (14.3%) primary site was unknown. Five patients had metastatic disease (71.4%), of which all involved the liver (71.4%). Supra-hilar lymph node recurrence was seen in 1 patient (14.3%). Six patients (85.7%) received treatment prior to SBRT, consisting of surgery (2 patients; 28.6%), somatostatin analogs (3 patients; 42.9%), transarterial chemoembolization (TACE) (3 patients; 42.9%), chemotherapy (2 patients, 28.6%).
Of the 5 patients with distant disease at diagnosis, SBRT was used for local control for enlarging primary tumors in the setting of stable distant disease. SBRT dosing consisted of 42–60 Gy over 3–8 fractions.
At the median follow up time of 13.8 months (range, 8.9–25.7 months), local control was achieved in all 8 (100%) PC tumor sites. A CR was achieved in 1 patient (14.3%), PR was seen in 5 patients (71.4%), and 1 (14.3%) patient had SD.
Two (28.6%) patients have since died of distant disease. There were no adverse events reported from this treatment modality.
Discussion
Traditionally, treatment for PC has been surgical resection, as complete resection often provides excellent PFS rates of 97% for TC and 80% for AC (11). Surgery may not be a possibility for every patient, as personal choice, previous surgeries, the presence of metastatic disease or comorbidities may preclude this treatment option for a select population.
In the management of NSCLC, surgical resection is also the preferred treatment for medically operable patients (12). Given that many patients who develop NSCLC have significant pulmonary or medical comorbidities, the alternative treatment to definitive surgery was historically fractionated radiotherapy. The Radiation Therapy Oncology Group (RTOG) trial 0236 tested the efficacy of SBRT in the treatment of early-stage NSCLC for medically inoperable patients (7). SBRT reduced overall treatment times from 30–40 daily treatments down to 3 treatments using focused, high-dose radiation to treat the tumor. The result was an increase in local control to 93% at 5 years and doubling of median overall survival to 48 months compared with historical outcomes. A more recent randomized trial comparing SBRT to standard fractionated radiation in patients with stage I peripheral NSCLC observed a significant reduction in local failure with SBRT [hazard ratio (HR) 0.32 (95% CI: 0.13–0.77); P=0.008] with 2-year local control in patients treated with SBRT 89% versus 65% in patients treated with standard radiation therapy (13). These results demonstrate that in early-stage NSCLC, SBRT provides a non-invasive, effective alternative to surgical resection for patients who are medically inoperable or refuse surgery.
Radiation therapy has not been typically utilized in PC; thus, the literature on this topic is sparse. Okoye et al. conducted a retrospective analysis examining treatment options in 52 patients with PCs. Only one of these patients received SBRT (14). Colaco et al. presented the first case series looking at SBRT in 4 patients with localized PC, which found that radiation offered good local control and the authors concluded that this treatment modality should be considered in those patients in which surgery is not an option (6). The second case series was published by Singh et al. examining SBRT in 10 patients with localized PC. Patients who had high grade tumors or metastatic disease were excluded. In line with the conclusions drawn by Colaco et al., Singh and colleges determined that SBRT offered promising control rates and survival data. Both of these studies report on localized PC, utilizing SBRT for definitive treatment (6,10).
Wegner et al. presented a large retrospective study comparing the effects of SBRT and conventionally fractionated radiotherapy (CFRT) on early-stage PC (9). This study utilized the National Cancer Database to identify 154 patients with typical PC staged as T1–2N0M0 treated non-surgically with SBRT (84 patients; 55%) or CFRT (70 patients; 45%. This study concluded that SBRT was associated with an improved overall survival when compared to CFRT in this patient population (66 vs. 58 months; P=0.034) (9). The role of SBRT in advanced disease was not explored in this study.
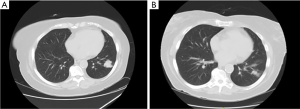
To the best of our knowledge, there has not been a study examining the effect of SBRT on patients with advanced or metastatic PC disease. Our study is the first known case series that examined the role of SBRT on a population with a majority being metastatic PCs. The data that we present is not only novel but important, as patients with PCs are not limited to early-stage disease. Additionally, patients who have metastatic PCs may have excellent control at distant metastasis through other treatment modalities, for example TACE for liver metastasis, but have a growing primary site disease. This was the case for 5 of our patients, all of whom achieved localized control of their lung lesion after the utilization of SBRT. Figure 1 is an example of response to SBRT in one of our patients.
Surgery should continue to be the treatment of choice in PCs; however, SBRT may offer an alternative choice for those who are not a surgical candidate or who do not wish to pursue surgical interventions. Furthermore, prospective studies should be conducted. Given the scarcity of this tumor, however, prospective studies are difficult to conduct. Data obtained from one nonrandomized study and a few case series make it challenging to draw specific treatment recommendations in non-surgical candidates. Additionally, utilizing SBRT to provide local control in metastatic disease has not been adequately explored.
Conclusions
We present this first known case series examining the effect of SBRT in PCs in both localized and metastatic disease. Our study shows that SBRT is a safe treatment option for patients with PC tumors managed in the non-surgical setting. SBRT in PCs provides excellent local control, in both early stage and in advanced or metastatic disease. It is reasonable to consider SBRT in patients with PCs who are ineligible for surgical resection due to comorbidities, tumor burden, personal preference, or advanced disease. Prospective studies are necessary to further consider the role of SBRT in PC tumors.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the AME Case Series reporting checklist. Available at http://dx.doi.org/10.21037/pcm-20-71
Data Sharing Statement: Available at http://dx.doi.org/10.21037/pcm-20-71
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pcm-20-71). RAR has served as a consultant or advisory board member for Ipsen Biophamaceuticals Inc., Advanced Accelerator Applications, Curium and Novartis Pharmaceuticals, Corp. as well as a speaker for Merck & Co. Inc., Genentech, Astra Zeneca, Guardant and Ipsen Biopharmaceuticals. He has received research funding from Aadi Bioscience and Merck & Co. J. Philip Boudreaux serves as a speaker and consultant for Ipsen Biopharmaceuticals, Inc., and Lexicon Pharmaceuticals, Inc., and as a speaker for Novartis Pharmaceuticals, Corp. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study received approval from Louisiana State University Health Sciences Center-New Orleans and Ochsner Clinic Foundation’s Institutional Review Boards (IRB# 987; 2011.038.C). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hemminki K, Li X. Incidence trends and risk factors of carcinoid tumors: a nationwide epidemiologic study from Sweden. Cancer 2001;92:2204-10. [Crossref] [PubMed]
- Travis WD, Brambilla E, Müller-Hermelink HK, et al. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. 1st edition. World Health Organization Classification of Tumours, 2004.
- Pieterman CR, Conemans EB, Dreijerink KM, et al. Thoracic and duodenopancreatic neuroendocrine tumors in multiple endocrine neoplasia type 1: natural history and function of menin in tumorigenesis. Endocr Relat Cancer 2014;21:R121-42. [Crossref] [PubMed]
- Gustafsson BI, Kidd M, Chan A, et al. Bronchopulmonary neuroendocrine tumors. Cancer 2008;113:5-21. [Crossref] [PubMed]
- García-Yuste M, Matilla JM, Cueto A, et al. Typical and atypical carcinoid tumours: analysis of the experience of the Spanish Multi-centric Study of Neuroendocrine Tumours of the Lung. Eur J Cardiothorac Surg 2007;31:192-7. [Crossref] [PubMed]
- Colaco RJ, Decker RH. Stereotactic radiotherapy in the treatment of primary bronchial carcinoid tumor. Clin Lung Cancer 2015;16:e11-4. [Crossref] [PubMed]
- Timmerman RD, Hu C, Michalski JM, et al. Long-term Results of Stereotactic Body Radiation Therapy in Medically Inoperable Stage I Non-Small Cell Lung Cancer. JAMA Oncol 2018;4:1287-8. [Crossref] [PubMed]
- Hendifar AE, Marchevsky AM, Tuli R. Neuroendocrine Tumors of the Lung: Current Challenges and Advances in the Diagnosis and Management of Well-Differentiated Disease. J Thorac Oncol 2017;12:425-36. [Crossref] [PubMed]
- Wegner RE, Abel S, Horne ZD, et al. Stereotactic body radiation therapy versus fractionated radiation therapy for early-stage bronchopulmonary carcinoid. Lung Cancer Manag 2019;8:LMT14. [Crossref] [PubMed]
- Singh D, Chen Y, Cummings MA, et al. Inoperable Pulmonary Carcinoid Tumors: Local Control Rates With Stereotactic Body Radiotherapy/Hypofractionated RT With Image-Guided Radiotherapy. Clin Lung Cancer 2019;20:e284-90. [Crossref] [PubMed]
- Ferguson MK, Landreneau RJ, Hazelrigg SR, et al. Long-term outcome after resection for bronchial carcinoid tumors. Eur J Cardiothorac Surg 2000;18:156-61. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN Guidelines for NSCLC v8.2020. 2020; Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed Sept 29, 2020.
- Ball D, Mai GT, Vinod S, et al. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): a phase 3, open-label, randomised controlled trial. Lancet Oncol 2019;20:494-503. [Crossref] [PubMed]
- Okoye CC, Jablons DM, Jahan TM, et al. Divergent management strategies for typical versus atypical carcinoid tumors of the thoracic cavity. Am J Clin Oncol 2014;37:350-5. [Crossref] [PubMed]
Cite this article as: Thomas K, Smith C, Marsala A, Boudreaux JP, Thiagarajan R, Ramirez RA. The use of stereotactic body radiotherapy in pulmonary carcinoid tumors: a case series. Precis Cancer Med 2021;4:11.