Unusual pattern of recurrence and atypical visceral metastases in extremity soft tissue sarcoma: a case report
Introduction
Soft-tissue sarcomas (STS) are a rare and heterogeneous group of malignancies with different prognosis depending on histological subtype, stage and anatomic location (1). Commonly, STS arise in the extremities, predominantly in the lower limbs, accounting for up to 50% of all STS, and the most frequent histological subtypes are liposarcoma and leiomyosarcoma followed by fibrosarcoma. Fibrosarcoma is composed of malignant spindle-cell fibroblasts and represents up to 5.2% of adult’s STS (2).
Multimodal treatment with limb sparing surgery combined with pre- or post-operative radiotherapy can lead to 5-years local control rates of up to 85–90% (3). Prognostic factors have been related to local failure risk and development of distant metastases, including increasing tumor size (>5 cm), high grade, positive surgical margin or depth to muscular fascia (4,5). The 5-year distant failure rate for patients with lower extremity STS is 30% being the most common site the lungs (6-9). Nonetheless, STS dissemination may also involve lymph nodes, the bone or the abdominal cavity. Intra-abdominal metastases from STS are extremely rare and occur mainly in the liver, being frequently observed in leiomyosarcoma (10-12).
Herein, we report the case of a patient diagnosed with lower extremity fibrosarcoma that underwent multimodal treatment, and then presented a series of loco-regional, outfield recurrences, followed by contralateral limb failure along with lung and gastric metastases.
We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/pcm-20-49).
Case presentation
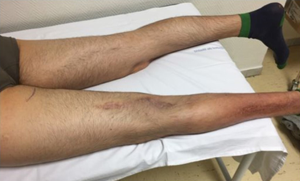
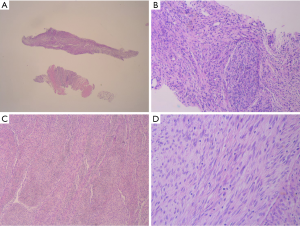
At the time of initial diagnosis, the patient was a 30-year-old male with no medical history of interest. He initially noticed a painful mass on the left calf, consulting an orthopedic, who ordered a magnetic resonance imaging (MRI) revealing a lesion within the left limb (Figure 1). In February 2013, patient underwent gross total resection with clear margins. Histological sections of the specimen showed spindle cell proliferation with two areas of tumor necrosis (5%). Immunohistochemical (IHC) analysis confirmed the presence of cells with positive only Vimentin marker (Figure 2). Based on these features the diagnosis established was Adult Fibrosarcoma (pT2b). Following the tumor board decision, the patient then completed treatment with adjuvant radiotherapy, up to a total dose of 66 Gy. First follow-up MRI, performed after 12 weeks of treatment, showed post-treatment changes with no findings suggestive of malignity. Patient at this time was asymptomatic and follow-up was continued during the next year every 6 months.
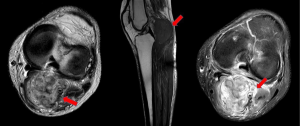
In September 2014, patient consults again after noticing a second mass, in the same limb, but in the left popliteal fossa area. A core needle biopsy of such node was performed and it was informed as a grade II fibrosarcoma. Case was presented in the multidisciplinary musculoskeletal tumor board, deciding neo-adjuvant radiotherapy up to 46 Gy, followed by total resection and intraoperative radiotherapy as a tumor bed boost.
After 17 months of this last approach, a third marginal recurrence presents on left thigh with a maximum diameter of 3 cm and aggressiveness criteria by MRI. Patient underwent total resection of the lesion with clear margins, followed by adjuvant radiotherapy and in addition, adjuvant chemotherapy, epirubicin and ifosfamide regimen. Histological specimen showed grade III fibrosarcoma with necrosis (80%). In the IHC analysis, positive Vimentin marker, Desmine, CD68 and factor XIIa marker, with a Ki- 67% of 20%. Patient received last cycle on December 2016, and initiated surveillance with lower extremities MRI and total body computed tomography (CT) every 4 months.
MRI of June 2018 described a new solid nodular lesion within the contralateral extremity soleus muscle. The total body CT also revealed recent increase in the size of pulmonary lesions described in previous imaging as nonspecific nodes. Patient received multidisciplinary management with neo-adjuvant radiotherapy followed by surgery and intraoperative radiotherapy tumor bed boost. In a second time, a wedge resection of the superior and inferior right pulmonary lobes was performed. The histological examination revealed no evidence of malignancy of pulmonary nodes but confirmation of recurrent high grade fibrosarcoma in the lower right extremity. With this final pathological anatomy report, and after radical treatment of the soft-tissue recurrence site, systemic treatment was not recommended at this time.
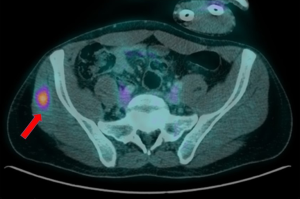
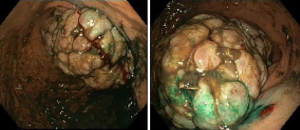
In July 2019 patient presents to the emergency room with clinical features suggestive of upper gastrointestinal bleeding with minimum blood test alteration. Patient was under study for a new suspicious lesion within the contralateral gluteus, with hypermetabolic activity on PET-CT (Figure 3). Study was completed with a panendoscopy examination, revealing a gastric mass suggestive of primary malignancy versus secondary disease (Figure 4). In August 2019, surgeons performed a total gastrectomy with Roux-en-Y reconstruction, and in parallel, orthopedic surgeon performed a total resection of the gluteal lesion. Finally the pathology report informed of a fibrosarcoma gastric metastasis with no lymph node involvement (25 nodes), necrosis <5% and a right gluteal metastasis. In the IHC analysis, positive Actin ML and HHF35, Desmine, and negative for Myogenin, with a Ki-67% of 80%. Prior to initiate systemic treatment, re-staging imaging revealed evidence of lung progression and a suspicious pancreatic lesion. Patient starts chemotherapy in October 2019, initially with adriamycin followed then by liposomal doxorubicin.
On his last visit, patient remains asymptomatic and at the time has received 5 cycles of doxorubicin. Last PET-CT (6th of February 2020) shows stability of bilateral pulmonary nodes with no evidence of new lesions and describes the pancreatic lesion as a possible pseudocyst. Therefore, treatment is discontinued for now, and patient initiates new follow-up plan with imaging studies every 3–4 months.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient. A patient perspective letter is added as supplementary file. In the attached file, the patient explains how he has experienced his disease during the 7 years of evolution, his mindset on the diagnostic tests and treatments, and the significant impact on his quality of life.
Discussion
Soft tissue sarcomas (STS) represent less than 1% of all neoplasms and include a wide variety of histological subtypes (2). Anatomically, extremities are the most common primary site, and generally lesions are found in deep soft tissue or adjacent to the bone (1,2). Local control depends on tumor grade and size, surgical margins and adjuvant radiotherapy. Extremity STS can achieve 5-year local control rates of up to 85–90%, ideally after a multimodal approach with radiotherapy followed by limb sparing surgery (3,13,14). There is no statistical difference in terms of local control between the different STS histological subtypes, nor is there evidence in the literature of a greater risk of marginal recurrences along the extremity in fibrosarcoma (2).
Previous series of extremity STS, document a mean time to local failure that may vary from 9 to 53 months (7,9,14). In the present case, as the patient had a high grade, deep tumor, with dimensions greater than 5 cm, the risk of local recurrence was higher. He received multimodal treatment with surgery and radiotherapy. Despite this, within a period of time shorter than 48 months, he developed three recurrences along the limb distal-proximal axis (Figure 5). These were considered marginal recurrences, outside the previous irradiation field, in the immediately superior area, allowing surgical salvage therapy and delivery of optimal doses of radiotherapy without field overlap.
Leibel et al. carried out a study which included 81 patients with extremity STS. Patients were treated with surgery, radiotherapy or both (13). With a mean time of 17 months, local failure was 41% after surgery alone, 100% after radiotherapy alone and 16% after surgery and radiotherapy (1/3 infield recurrence). Distant metastases occurred during the first two years after treatment for the majority of patients, being the most common site the lung (90%).
In a more recent study, that included 174 patients with localized extremity STS, patterns of recurrence were analyzed in order to establish routine follow-up (14). After multimodal treatment with surgery (n=172), radiotherapy (n=145) and chemotherapy (n=25) the 5-year local control rate was 51%. With a mean time of 9 months, 31 patients presented local failure, having 5 of them synchronous metastases. Distant disease occurred in 51 patients, with a mean time of 5 months for lung failure and 11 months for non-pulmonary metastasis.
Similarly, Dogan et al, studied long-term outcomes in 114 patients with primary extremity STS all treated with surgery and radiotherapy followed by adjuvant chemotherapy in 43 cases (9). The 5-year and 10-year local control rates were 77% and 70% respectively with a mean time of 53 months to local recurrence. Five-year and 10-year disease-free survival was 60% and 52% respectively, with up to 27% of distant relapse, more frequently in lungs.
In the reviewed literature, there is no evidence of the effect of local control on the development of distant metastases. Smith et al. analyzed the patterns of relapse in extremity STS, observing that up to 80% of patients with distant failure presented lung metastasis and these appeared after a mean time of 20.5 months (7). In the case we have reported, distant failure wasn’t observed until 60 months after primary site treatment, and presented with a synchronous contralateral limb metastases.
Although the majority of patients with distant failure developed lung metastases, other sites of dissemination such as bone or abdominal cavity have also been described. Within STS, intra-abdominal metastases may develop more frequently in myxoid liposarcoma, but for other histological subtypes the incidence is low, ranging from 1–6% (15,16). The overview of previous studies reporting for STS abdominal metastases is provided in Table 1.
Table 1
| Study (year of publication) | Patients with AM from STS | AM location (n) | Patients with previous or synchronous non-AM | Mean time to AM | Management (n) | Prognosis |
|---|---|---|---|---|---|---|
| Ogose et al. (10), 2000 | Primary site: Extremities [14]; Trunk wall [1]; Abdomen/pelvis *[8] | Visceral: Liver [14]; Jejunum [2]; Pancreas [1]; Colon [1]; Jejunum and pancreas [1]; Other: Peritoneum [4]; | 22; Lung [19]; Other: Bone, soft tissue and brain | Liver: 17.6 months; Other: 19.4 months | Conservative treatment [18]; Surgery [1]; RT [1] | 23 pts DOD; Mean time to event: 8.2 months |
| Behranwala et al. (15), 2004 | Primary site: Extremities [18]; Trunk wall [1] | Visceral: Gastric [1]; Small bowel [4]; Large bowel [1]; Liver [1]; Spleen [1]; Adrenal [2]; Other: Omentum [3]; Mesentry [9]; Retroperitoneal [8]; | 13; Lung [6]; Other: Soft tissue | 27 months | Surgery [16]; Plus adjuvant RT and CT [3] | 2-year post-AM; survival was 54% surgical treatment; and 0% for unresected patients |
| Sheah et al. (17), 2008 | Primary site: Extremities [6]; Abdomen/pelvis * [1] | Visceral: Liver [4]; Other: Retroperiteal [1]; Mesentery/soft-tissue [2]; | 4; Lung [2]; Other: Bone and soft tissue | 2.7 years | N/A | N/A |
| King et al. (16), 2009 | Primary site: Extremities [10] | Visceral: Liver [2]; Adrenal [1]; Other: Intraabdominal [3]; Retroperitoneal [2]; Abdomen wall [1]; Widespread [1] | 9; All lung | 19.8 months; *4 patients AM at diagnosis; | N/A | At time of study: 7 pts DOD; 3 pts. AWD |
| Rehders et al. (12), 2009 | Primary site: Extremities [5]; Abdomen/pelvis *[22] | Liver [27] | 4; Lung [2]; Nodal [2] | 44 months | All surgery; Plus adjuvant CT [4] | 5-year and 10-year post-AM survival were 49% and 33%, respectively |
| Thompson et al. (18), 2015 | Primary site: Extremities [7] | Visceral: Liver [4]; Other: Abdominal [1]; Mesenteric [1]; Peritoneal [1] | 5; All lung | 27.9 months | N/A | N/A |
| Grimme et al. (19), 2019 | Primary site: Extremities [5]; Abdomen/pelvis* [22]; Head&neck [2]; Other [9]; | Liver [38]; 51% solitary | 12 | N/A; 11 pts. Synchronous with primary tumor | All surgery; | 3-year and 5-year post-AM survival were 53.9 and 41.1% respectively |
| Smolle et al. (11), 2020 | Primary site: Extremities [24]; | Visceral: Liver [13]; Intestinal [7] Pancreatic [2]; Other: Peritoneal [2] | 14 | 2.4 years | CT [19]; Surgery and CT [2]; Supportive care [1] | 2-year post-AM survival was 60–65% |
*, Abdomen/pelvis: viscera, retroperitoneum, gynaecologic, pelvis. AM, abdominal metastases; DOD, dead of disease; AWD, alive with disease.
Smolle et al. carried out a multicenter study that included up to 796 patients with localized STS mainly of the extremities (11). Up to 202 patients presented distant failure, but just 24 of these had intra-abdominal metastasis. The mean time to abdominal recurrence was 2.4 years, and the most common site was the liver. Myxoid liposarcoma followed by pleomorphic liposarcoma were the histological subtypes with higher abdominal affinity. Undifferentiated tumors also presented higher risk of developing primary abdominal metastases, although this was not significant in multivariate analysis (P=0.277).
Regarding primary site location, extremities STS tend to have more abdominal distant failure compared to trunk wall or head and neck sites. Two retrospective series analyzing surgical treatment for liver metastases from STS, found that by far the most common primary site was the abdomen and pelvis (12,19). However, other series that analyze the presence of intra-abdominal metastases, predominantly include extremities STS with secondary tumor sites such as liver, bowel, spleen and also non-visceral abdominal spread (10,15,17).
Little is known about gastric metastases from STS, as they are mainly documented in case reports or as isolated cases in larger studies (15,20). To the best of our knowledge, our case report is the first one to document a gastric lesion from an extremity fibrosarcoma. As shown in Table 1, the treatment of choice for abdominal metastases tends to be surgery followed in some cases by adjuvant therapy. This radical approach in patients with distant disease provides a greater disease-free interval and 2-year post-metastasis survival rate (12,19).
Myxoid liposarcomas differ from other subtypes, as they show a tendency toward extrapulmonary spread and therefore seem to benefit from a more rigorous follow-up with whole body CT or MRI, for early detection of abdominal dissemination (16,17). However, this intensified follow-up regimen has not been considered for other STS subtypes such as fibrosarcoma. Regular surveillance strategies for extremity STS, consists of a 3-monthly physical examination and involved extremity MRI or ultrasound and a chest CT or X-ray, every 3–6 months for the first 2–3 years as the majority of STS relapse occur within the first 3 years after completion of treatment (8). Nonetheless, some doubts arise regarding the utility of MRI of the primary involved area during follow-up, as more than 95% of local recurrences are noted clinically by physician or the patient themselves (14,21,22). This is consistent with our findings, as all of the repeated recurrences located in the left limb and in the contralateral limb, were identified by the patient. Regarding chest imaging, a randomized trial comparing standard follow-up showed non-inferiority of chest X-ray compared to CT scan. Although CT scans offer an earlier detection of pulmonary lesions, this does not lead to an improvement in terms of survival compared to chest X-ray (21).
In relation to STS follow-up after distant failure, there is no consensus, however based on synchronous stage IV STS follow-up recommendations, we can conclude that chest, and other known sites of metastatic disease, imaging (CT preferred) every 2–6 months for 2–3 years and then every 6 months for the next 2 years, seems a safe approach (22). However, there is no evidence, of the benefit of routine abdominal-pelvic imaging for fibrosarcoma STS.
In summary, high-risk STS patients generally relapse within the first 2–3 years, usually with pulmonary metastases or/and local recurrence, however gastric dissemination is uncommon. To our knowledge, this is the first case of STS fibrosarcoma with gastric metastases published in the literature. Surveillance strategies should therefore focus on the individual risk of each patient.
One of the main strengths of this study, is its long clinical evolution of more than five years, revealing the therapeutic effort with local treatments such as surgery or radiotherapy. However, there are some limitations inherit to the type of publication, single case study, not being therefore able to recommend what would be the ideal follow-up strategy in patients with STS after local and distant failure.
Conclusions
The case reported highlights the importance of patient’s self-examination for local recurrence detection. Multimodal management for primary site is the key to achieve high rates local control and long disease-free survival. Local radical approaches to secondary sites should be considered for selected patients with STS.
Acknowledgments
The authors thank Christian Fernandez MD, for his critical proof reading and language assistance.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/pcm-20-49
Peer Review File: Available at http://dx.doi.org/10.21037/pcm-20-49
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pcm-20-49). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient. Patient was offered the opportunity to read the manuscript.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fletcher CD, Bridge JA, Hogendoorn PC, et al. Pathology and Genetics of Tumours of Soft Tissue and Bone 4th. Lyon, France: IARC Press; WHO Classification of Tumours of Soft Tissue and Bone 2013:91-2.
- Toro JR, Travis LB, Wu HJ, et al. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978–2001: an analysis of 26,758 cases. Int J Cancer 2006;119:2922-30. [Crossref] [PubMed]
- Beane JD, Yang JC, White D, et al. Efficacy of adjuvant radiation therapy in the treatment of soft tissue sarcoma of the extremity: 20-year follow-up of a randomized prospective trial. Ann Surg Oncol 2014;21:2484-9. [Crossref] [PubMed]
- Maretty-Nielsen K, Aggerholm-Pedersen N, Safwat A, et al. Prognostic factors for local recurrence and mortality in adult soft tissue sarcoma of the extremities and trunk wall: a cohort study of 922 consecutive patients. Acta Orthop 2014;85:323-32. [Crossref] [PubMed]
- Zagars GK, Ballo MT, Pisters PW, et al. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: An analysis of 1225 patients. Cancer 2003;97:2530-43. [Crossref] [PubMed]
- Trovik CS, Bauer HC, Alvegård TA, et al. Surgical margins, local recurrence and metastasis in soft tissue sarcomas: 559 surgically-treated patients from the Scandinavian Sarcoma Group Register. Eur J Cancer 2000;36:710-6. [Crossref] [PubMed]
- Smith HG, Memos N, Thomas JM, et al. Patterns of disease relapse in primary extremity soft-tissue sarcoma. Br J Surg 2016;103:1487-96. [Crossref] [PubMed]
- Casali PG, Abecassis N, Aro HT, et al. Soft tissue and visceral sarcomas: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv267-9. [Crossref] [PubMed]
- Dogan ÖY, Oksuz DÇ, Atalar B, et al. Long-term results of extremity Soft Tissue Sarcomas Limb-sparing Surgery and Radiotherapy. Acta Ortop Bras 2019;27:207-11. [Crossref] [PubMed]
- Ogose A, Morita T, Hotta T, et al. Intra-abdominal metastases in musculoskeletal sarcomas. J Orthop Sci 2000;5:463-9. [Crossref] [PubMed]
- Smolle MA, Schaffler A, Leithner A, et al. Incidence, treatment and outcome of abdominal metastases in extremity soft tissue sarcoma: Results from a multi-centre study. J Surg Oncol 2020;121:605-11. [Crossref] [PubMed]
- Rehders A, Peiper M, Stoecklein NH, et al. Hepatic metastasectomy for soft-tissue sarcomas: is it justified? World J Surg 2009;33:111-7. [Crossref] [PubMed]
- Leibel SA, Tranbaugh RF, Wara WM, et al. Soft Tissue Sarcomas of the Extremities Survival and Patterns of Failure with Conservative Surgery and Postoperative Irradiation Compared to Surgery Alone. Cancer 1982;50:1076-83. [Crossref] [PubMed]
- Rothermundt C, Whelan JS, Dileo P, et al. What is the role of routine follow-up for localised limb soft tissue sarcomas? A retrospective analysis of 174 patients. Br J Cancer 2014;110:2420-6. [Crossref] [PubMed]
- Behranwala KA, Roy P, Giblin V, et al. Intra-abdominal metastases from soft tissue sarcoma. J Surg Oncol 2004;87:116-20. [Crossref] [PubMed]
- King DM, Hackbarth DA, Kilian CM, et al. Soft-tissue sarcoma metastases identified on abdomen and pelvis CT imaging. Clin Orthop Relat Res 2009;467:2838-44. [Crossref] [PubMed]
- Sheah K, Ouellette HA, Torriani M, et al. Metastatic myxoid liposarcomas: imaging and histopathologic findings. Skeletal Radiol 2008;37:251-8. [Crossref] [PubMed]
- Thompson MJ, Ross J, Domson G, et al. Screening and surveillance CT abdomen/pelvis for metastases in patients with soft-tissue sarcoma of the extremity. Bone Joint Res 2015;4:45-9. [Crossref] [PubMed]
- Grimme FAB, Seesing MFJ, van Hillegersberg R, et al. Liver Resection for Hepatic Metastases from Soft Tissue Sarcoma: A Nationwide Study. Dig Surg 2019;36:479-86. [Crossref] [PubMed]
- Lee GW, Kim TH, Min HJ, et al. Unusual gastrointestinal metastases from an alveolar soft part sarcoma. Dig Endosc 2010;22:137-9. [Crossref] [PubMed]
- Puri A, Ranganathan P, Gulia A, et al. Does a less intensive surveillance protocol affect the survival of patients after treatment of a sarcoma of the limb? Updated results of the randomized TOSS study. Bone Joint J 2018;100-B:262-8. [Crossref] [PubMed]
- NCCN clinical practice guidelines in oncology: Soft Tissue Sarcoma. Version 1. 2021.
Cite this article as: Sedano P, González-San Segundo C, Rodríguez-Pertierra M, Díaz-Gutiérrez F, Medrano A, Agra-Pujol C. Unusual pattern of recurrence and atypical visceral metastases in extremity soft tissue sarcoma: a case report. Precis Cancer Med 2021;4:4.