
Management of acquired resistance to ALK inhibitors: repeat biopsy to characterize mechanisms of resistance does not significantly impact clinical outcomes
Introduction
ALK gene rearranged-positive NSCLCs are highly responsive to small-molecule tyrosine kinase inhibitors (TKIs) that target ALK (1,2). Numerous ALK inhibitors have been developed during the past decade. Standard treatment of this group of patients has recently shifted from sequential crizotinib, which is the first first-generation ALK inhibitor, followed by more potent second-generation ALK TKIs to front-line second-generation TKIs followed by lorlatinib which is the only third-generation ALK TKI currently (3-12).
To date, three second-generation ALK inhibitors (ceritinib, alectinib, and brigatinib) have received the approval by US FDA for front-line treatment of advanced ALK-positive NSCLC with clinical trials demonstrating remarkable response (6-9). Ensartinib, another second-generation ALK inhibitor, was also recently reported the efficacy over crizotinib in first-line therapy (13).
A third-generation ALK and ROS1 TKI, lorlatinib has shown activity against the majority of acquired resistance mutations within the ALK kinase domain, such as ALK G1202R, ROS1 G2032R (14,15). Results recently reported from the randomized phase III trial CROWN demonstrated significantly longer progression-free survival over crizotinib in the front-line setting (16).
The knowledge gained about resistance mechanisms has guided scientists in discoveries that led to the rapid journey of ALK-targeted drug development. With increasing treatment options, the decision of treatment sequence has become more and more complex in ALK-positive patients in order to achieve the longest survival outcome. This article provides the position that currently, the clinical benefit of treatment sequencing in this group of patients can be achieved regardless of rebiopsy procedures at the time of disease progression.
Resistance mechanisms of each second-generation ALK inhibitors as the front-line treatment
The second-generation ALK inhibitors (ceritinib, alectinib, brigatinib, and ensartinib) were initially designed to overcome the secondary mutations from crizotinib resistance, for example L1196M, or ALK G1202R mutation (17). Currently, they are approved to treat ALK-positive patients as the first-line treatment, except ensartinib which has not been approved yet.
Gainor et al. reported the acquired resistance mechanisms of second-generation ALK inhibitors. They found ALK resistance mutations in 54% of patients post-ceritinib treatment, in 53% of patients post-alectinib treatment, and in 71% of patients post-brigatinib treatment. The different spectra of the ALK-dependent resistance mutations were C1156Y, I1171T/N/S, F1174L/C, V1180L, L1196M, G1202R, G1202del, D1203N, S1206Y/C, and E1210K (Figure 1) (18). G1202R was found 21%, 29%, and 43% in post ceritinib, alectinib, and brigatinib, respectively (18). Indeed, consistent with preclinical data, ALKG1202R emerged as the most common ALK resistance mutation among patients receiving second-generation ALK inhibitors. These solvent-front mutations (G1202R, G1202del, D1203N, and S1206Y/C) impair drug binding through steric hindrance (19). Several studies have demonstrated that G1202R confers high-level resistance to first- and second-generation ALK inhibitors (18,19). The other resistance mutations affect residues adjacent to the N-terminus (C1156Y) and C-terminus of the αC-helix (F1174C/L/V) which may enhance the kinase’s ATP-binding affinity and increase its enzymatic activity (18,19). The I1171T/N/S mutations also distort the αC-helix to interfere with TKI binding (18,20). ALK amplification has not yet been reported as a resistance mechanism after second-generation ALK inhibitors (18). Drug efflux pump (P-glycoprotein; P-gp) overexpression is one of ALK-independent resistance mechanisms which could significantly limit the CNS penetration of crizotinib and ceritinib (21) whereas alectinib is not a P-gp substrate thus able to achieve higher CNS levels (21). Bypass track activation (EGFR, HER2, PIK3CA, CKIT, BRAF, DDR2, FGFR2, NRAS and MET alterations), together with lineage change by transforming to small cell lung cancer are also the ALK-independent (off-target) resistance mechanisms (18,22).
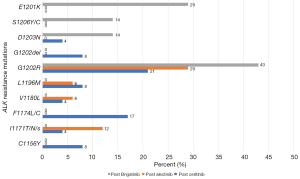
Regarding the structural differences among available ALK TKIs, each drug appears to be associated with a specific profile of secondary ALK resistance mutations. In the setting of second-generation ALK inhibitor failure, the development of secondary ALK resistance mutations imply that ALK may still be functioning as oncogenic driver. In the era when only first or second-generation ALK inhibitors were available in the market, there were several studies trying to show the efficacy of sequential treatment by another second-generation ALK TKIs in the setting of second-generation failure, but the overall response rate (ORR) (16–25%) and the PFS were limited (3–4 months) (23,24).
In patient-derived NSCLC cell lines with resistance to second-generation ALK TKIs, the third generation ALK inhibitor lorlatinib could inhibit the growth of cells harboring ALK resistance mutations with the lower of IC50 compared to other ALK inhibitors, but it was inactive against those cell lines without ALK resistance mutations (18).
Third-generation ALK inhibitor as the rescue drug
Lorlatinib is a reversible ATP-competitive third-generation ALK and ROS1 TKI. Lorlatinib has shown activity against the majority of known acquired ALK kinase domain resistance mutations from second-generation ALK TKI failure including the highly refractory ALK G1202R solvent front mutation (15,18). Furthermore, lorlatinib was specifically designed to penetrate CNS, thus it demonstrated better CNS response rates (in particular CNS complete responses) compared to the first-, and second-generation ALK inhibitors (16).
In the clinical phase II study of lorlatinib, there were 6 expansion cohorts using lorlatinib as the second- or later- line treatment. The cohort of patients with post-crizotinib failure (cohort 2 and 3A) demonstrated overall response rate (ORR) of 72.9%, and intracranial response rate (icORR) of 70.3% with median PFS of 11.1 months (11), whereas in the post non-crizotinib setting, either as the second- or late- line treatment (cohort 3B), lorlatinib showed ORR of 42.9% and icORR of 46.2%. Furthermore, the cohort of patients heavily pretreated by more than one ALK inhibitors (cohort 4–5) revealed an ORR of 39.6%, icORR of 48.1% and median PFS of 6.9 months (11). Lorlatinib was active against all known ALK resistance mutations including G1202R both in single and compound mutations with an ORR of 57%, median duration of response (DOR) of 7 months and median PFS of 8.2 months (Table 1) (25). One third of patients in this study harbored compound ALK resistance mutations. The authors compared the efficacy of lorlatinib in second-generation ALK TKIs failure and heavily pretreated patients with either one ALK mutation or compound ALK mutations. Results showed higher ORR (75% vs. 56%), longer median DOR (24.4 vs. 6.1 months) in patients with only one ALK resistance mutation (25). Although tumor genotyping for ALK mutations in patients who had failed from a second-generation ALK inhibitor may predict the response from lorlatinib, the efficacy of lorlatinib in patients with post-crizotinib treatment was comparable between patients with and without ALK mutations by using tissue, or plasma genotyping (25).
Table 1
| ALK resistance mutations | N | ORR % (95% CI) | Median PFS Months (95% CI) |
|---|---|---|---|
| G1269A | 9 | 89% (52–100%) | NR (8.2–NR) |
| I1171X | 8 | 75% (35–97%) | 5.5 (4.1–6.9) |
| L1196M | 12 | 67% (35–90%) | NR (2.8–NR) |
| G1202R/del | 28 | 57% (37–76%) | 8.2 (5.6–25.6) |
| F1174X | 12 | 42% (15–72%) | 7.4 (2.8–NR) |
ORR, overall response rate; NR, not reach.
A recent huge multicentre real-world data with a total of 76 advanced ALK-positive NSCLC patients, among patients treated with less than two previous TKIs, two or more previous TKIs, and three or more previous TKIs, the ORR and mPFS were 42% and not reached (NR), 35% and 11.2 months,18% and 6.5 months, respectively. The ORR and mPFS were 13% and 9.2 months for patients treated with one second-generation ALK inhibitor as the only ALK TKI received. The icORRs were 35% for 52 ALK-positive patients and the mPFS was 9.3 months for patients with leptomeningeal carcinomatosis (26).
Based on the robust results of lorlatinib in several settings (post crizotinib, post second generation ALK inhibitors, and heavily pretreated patients with more than one ALK TKIs), lorlatinib has been approved by US FDA for treatment in ALK-positive metastatic NSCLC patients who were previously treated with ≥1 ALK inhibitors without requirement for biomarker testing to demonstrate specific resistance mutations, unlike the initial osimertinib approval as second-line treatment predicated on finding the EGFR T790M resistance mutation after 1st or 2nd generation EGFR TKI therapy.
Resistance after lorlatinib failure
Lorlatinib-resistant molecular profile was explored at Massachusetts General Hospital (MGH) in 20 heavily pre-treated patients with lorlatinib treatment failure. The investigators found 50% of compound ALK resistance genes, with either double or triple mutations in acquired resistance to lorlatinib. ALK-independent mechanisms of resistance were thought to mediate resistance in patients who did not demonstrate any ALK resistance mutation. In addition, 2 patients’ tumors “lost” their lorlatinib-sensitive mutations (I1171N, G1202R) post-lorlatinib. There was no small cell transformation (27). Resistance to ALK inhibition is a dynamic and clonal process. In one patient case, a founder ALK C1156Y clone in the lorlatinib pretreatment tumor led to a double-mutant subclone (ALK C1156Y–L1198F) in the post-lorlatinib tumor upon disease progression (28). This acquired L1198F mutation conferred resistance to lorlatinib, but unexpectedly restored sensitivity to crizotinib, a less potent and less selective first-generation inhibitor. These results highlight the usefulness of developing multiple, structurally distinct inhibitors that target the same oncogenic kinase domain. When resistance develops to third-generation TKI, repeat biopsy can provide crucial information as to whether sequential treatment with a different ALK inhibitor may be also effective afterward.
Conclusions
The current therapeutic paradigm for advanced ALK-positive NSCLC is to treat with sequential ALK inhibitors, nowadays starting with one of the second-generation ALK TKIs as the first-line treatment then follow by third-generation ALK inhibitor, instead of starting with first-generation ALK TKI. Although this treatment approach has dramatically improved the survival of patients, third-generation acquired resistance invariably develops and leads to clinical progression. As lorlatinib potentially moves into the first-line setting, rebiopsy to characterize resistance mechanisms might be necessary to determine the role of earlier generation ALK TKIs in this setting. However, re-biopsy at the time of 1st or second-generation treatment failure to characterize mechanisms of resistance does not significantly impact clinical outcomes at this time as discussed above.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor Grace K. Dy for the series “Evidence and Controversies in the treatment of metastatic NSCLC” published in Precision Cancer Medicine. The article has undergone peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pcm-2020-mnsclc-09). The series “Evidence and Controversies in the treatment of metastatic NSCLC” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. [Crossref] [PubMed]
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [Crossref] [PubMed]
- Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in Patients With Crizotinib-Refractory Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Randomized, Multicenter Phase II Trial. J Clin Oncol 2017;35:2490-8. [Crossref] [PubMed]
- Ou SH, Ahn JS, De Petris L, et al. Alectinib in Crizotinib-Refractory ALK-Rearranged Non-Small-Cell Lung Cancer: A Phase II Global Study. J Clin Oncol 2016;34:661-8. [Crossref] [PubMed]
- Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014;370:1189-97. [Crossref] [PubMed]
- Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389:917-29. [Crossref] [PubMed]
- Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet 2017;390:29-39. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus Crizotinib in ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2027-39. [Crossref] [PubMed]
- Shaw AT, Felip E, Bauer TM, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol 2017;18:1590-9. [Crossref] [PubMed]
- Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol 2018;19:1654-67. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Horn L. Phase III Randomized study of ensartinib versus crizotinib in anaplastic lymphoma kinase (ALK)-positive NSCLC patients (eXalt3). The International Association for the Study of Lung Cancer, World Conference on Lung Cancer Virtual Presidential Symposium; 8 August 2020; Denver, USA2020.
- Johnson TW, Richardson PF, Bailey S, et al. Discovery of (10R)-7-amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-8,4-(m etheno)pyrazolo[4,3-h][2,5,11]-benzoxadiazacyclotetradecine-3-carbonitrile (PF-06463922), a macrocyclic inhibitor of anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) with preclinical brain exposure and broad-spectrum potency against ALK-resistant mutations. J Med Chem 2014;57:4720-44. [Crossref] [PubMed]
- Zou HY, Friboulet L, Kodack DP, et al. PF-06463922, an ALK/ROS1 Inhibitor, Overcomes Resistance to First and Second Generation ALK Inhibitors in Preclinical Models. Cancer Cell 2015;28:70-81. [Crossref] [PubMed]
- Shaw AT, Bauer TM, de Marinis F, et al. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N Engl J Med 2020;383:2018-29. [Crossref] [PubMed]
- Qiao H, Lovly CM. Cracking the Code of Resistance across Multiple Lines of ALK Inhibitor Therapy in Lung Cancer. Cancer Discov 2016;6:1084-6. [Crossref] [PubMed]
- Gainor JF, Dardaei L, Yoda S, et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov 2016;6:1118-33. [Crossref] [PubMed]
- Ignatius Ou SH, Azada M, Hsiang DJ, et al. Next-generation sequencing reveals a Novel NSCLC ALK F1174V mutation and confirms ALK G1202R mutation confers high-level resistance to alectinib (CH5424802/RO5424802) in ALK-rearranged NSCLC patients who progressed on crizotinib. J Thorac Oncol 2014;9:549-53. [Crossref] [PubMed]
- Katayama R, Friboulet L, Koike S, et al. Two novel ALK mutations mediate acquired resistance to the next-generation ALK inhibitor alectinib. Clin Cancer Res 2014;20:5686-96. [Crossref] [PubMed]
- Kort A, Sparidans RW, Wagenaar E, et al. Brain accumulation of the EML4-ALK inhibitor ceritinib is restricted by P-glycoprotein (P-GP/ABCB1) and breast cancer resistance protein (BCRP/ABCG2). Pharmacol Res 2015;102:200-7. [Crossref] [PubMed]
- Miyamoto S, Ikushima S, Ono R, et al. Transformation to small-cell lung cancer as a mechanism of acquired resistance to crizotinib and alectinib. Jpn J Clin Oncol 2016;46:170-3. [PubMed]
- Hida T, Seto T, Horinouchi H, et al. Phase II study of ceritinib in alectinib-pretreated patients with anaplastic lymphoma kinase-rearranged metastatic non-small-cell lung cancer in Japan: ASCEND-9. Cancer Sci 2018;109:2863-72. [Crossref] [PubMed]
- Lin JJ, Zhu VW, Schoenfeld AJ, et al. Brigatinib in Patients With Alectinib-Refractory ALK-Positive NSCLC. J Thorac Oncol 2018;13:1530-8. [Crossref] [PubMed]
- Shaw AT, Solomon BJ, Besse B, et al. ALK Resistance Mutations and Efficacy of Lorlatinib in Advanced Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2019;37:1370-9. [Crossref] [PubMed]
- Zhu VW, Lin YT, Kim DW, et al. An International Real-World Analysis of the Efficacy and Safety of Lorlatinib Through Early or Expanded Access Programs in Patients With Tyrosine Kinase Inhibitor-Refractory ALK-Positive or ROS1-Positive NSCLC. J Thorac Oncol 2020;15:1484-96. [Crossref] [PubMed]
- Yoda S, Lin JJ, Lawrence MS, et al. Sequential ALK Inhibitors Can Select for Lorlatinib-Resistant Compound ALK Mutations in ALK-Positive Lung Cancer. Cancer Discov 2018;8:714-29. [Crossref] [PubMed]
- Shaw AT, Friboulet L, Leshchiner I, et al. Resensitization to Crizotinib by the Lorlatinib ALK Resistance Mutation L1198F. N Engl J Med 2016;374:54-61. [Crossref] [PubMed]
Cite this article as: Prasongsook N, Reungwetwattana T. Management of acquired resistance to ALK inhibitors: repeat biopsy to characterize mechanisms of resistance does not significantly impact clinical outcomes. Precis Cancer Med 2021;4:8.

