
ADAURA: a definitive answer to the wrong question
Introduction
The ADAURA trial tested the utility of up to 3 years of osimertinib to significantly improve disease-free survival (DFS) in patients with resected stage IB–IIIA EGFR mutation-positive (EGFRm+) non-small cell lung cancer (NSCLC) who may have received prior adjuvant chemotherapy. This trial, with accrual terminated early at the behest of the Independent Data Monitoring Committee (IDMC) after an unplanned analysis revealed a highly significant difference in DFS at a point when patients had been on assigned therapy a median of approximately 22 months (1), was presented with great fanfare at the Plenary Session of the ASCO 2020 Annual Meeting (2) and recently published (3). There is no debate that the trial is positive, demonstrating a hazard ratio (HR) for DFS of 0.17 in patients with resected stage II–IIIA NSCLC and a HR for DFS of 0.21 for the broader enrolled population that included patients with stage IB NSCLC along with those with higher stage NSCLC. What should be debated, however, is whether this is a definitive answer to the wrong clinical question, as well as whether the inherent shortcomings of this trial’s execution undermine our ability to assess the true relevant question of whether adjuvant osimertinib improves overall survival (OS) in this population.
Improvement in DFS on ADAURA is neither surprising nor sufficient
That DFS was significantly improved in patients who are still in the midst of their treatment with an agent known to be highly effective for a prolonged duration in the advanced NSCLC setting is not remotely surprising. DFS has already been shown to be favorable in the SELECT (4) and RADIANT (5) trials, both evaluating adjuvant erlotinib and revealing a very promising pattern of DFS in EGFRm+ but no indication of improved OS. Instead, the prolonged follow-up of patients beyond the 2 years that patients received erlotinib demonstrated that many patients assigned to receive erlotinib demonstrated relapse in the months after discontinuing erlotinib (4). Similarly, the ADJUVANT/CTONG1104 trial of Chinese patients with stage IB–IIIA EGFRm+ NSCLC randomized between adjuvant conventional chemotherapy or gefitinib for up to 2 years demonstrated a significantly superior DFS with gefitinib (6) not accompanied by any improvement in OS (7).
Despite the fact that osimertinib has demonstrated significantly superior efficacy compared to the first generation EGFR tyrosine kinase inhibitors (TKIs) gefitinib and erlotinib (8), thus far we have only seen from ADAURA that it is possible to administer prolonged osimertinib to achieve at least a transient improvement in the appearance of scans, without any improvement in OS yet, with the counterbalance of knowingly overtreating some patients not just for a period of a few months but for many years with a therapy that has a financial cost in the range of $250,000/year, per patient in the US.
OS is the clear endpoint of choice
The critical questions we should be asking of adjuvant therapy are the following:
- Can administration eradicate the micrometastatic disease that is the presumed target of any adjuvant therapy, which would therefore cure patients who were otherwise destined to have their micrometastatic disease after surgery develop into grossly detectable relapse and subsequent death from disease progression?
- Failing that, does administration lead to recipients living significantly longer than an alternative strategy of treating only those whose disease relapses with timely, optimal therapy in that setting? Specifically, does adjuvant osimertinib lead to a significant improvement in OS compared to prompt treatment with osimertinib at the earliest evidence of relapse?
Unfortunately, the ADAURA trial was designed to surpass the far lower bar of significantly improving DFS. Tellingly, the thoracic oncology community never considered significant improvement in DFS to be an appropriate endpoint to change the standard of care when designing or reviewing the results of the many trials around the world that established the role of adjuvant chemotherapy, which are all predicated on a significant improvement in OS (9). Nor was DFS considered the appropriate primary endpoint in the more recently developed and completed ECOG1505 trial that tested the addition of bevacizumab to adjuvant chemotherapy (10).
Compared to endpoint of OS that we have historically sought, DFS clearly offers the advantage to sponsor companies of an earlier readout and a far lower hurdle to achieve a positive trial. However, the fact that the FDA and many academic oncologists now view DFS as an acceptable criterion for drug approval should not be interpreted as it reflecting a new gold standard, but rather an erosion of prior rigor demonstrated by these groups, perhaps a product of an affirming motivation to demonstrate “wins” in drug approvals, assisted by enthusiasm of both the FDA and investigators for the promise of targeted therapies for enriched populations. We should hope for the same rigor in testing newer approaches that we demanded of the treatments that have established our standards up to now.
The available results of several prior trials of adjuvant EGFR TKIs in molecularly selected patients highlight that DFS can be readily achieved in the absence of an improvement in OS (4-7). This pattern is prone to be demonstrated with a therapy that can provide marked benefit if given upon relapse, as targeted therapies typically do. DFS will emphasize a “front-loaded” benefit of early treatment that may potentially be achieved with subsequent treatment upon relapse only as needed later. In addition, inaccurate or suboptimal staging of patients on a trial intended for patients with early stage NSCLC creates a scenario that confers a marked advantage to proactive treatment in terms of DFS that may or may not translate to a subsequent benefit in OS.
Weak staging offers a marked bias in favor of proactive treatment
It is instructive here to consider the results of the aforementioned ADJUVANT/CTONG1104 trial of adjuvant gefitinib vs. chemotherapy, which revealed a steady decline in DFS in both groups by 4–5 years out, indicating that very few enrolled patients were cured after surgery in either arm (6,7). This is the pattern that would be expected if many patients were under-staged, as would be prone to occur in this study that did not require PET/CT, brain MRI imaging, or invasive mediastinal staging, despite a significant fraction of these patients having disease that was stage IIIA N2 based on CT imaging. This represents a problematic limitation in the interpretability of the trial that may also apply to ADAURA as well, as suggested by the high rate of relapse in the placebo arm that exceeds that of historical standards for this population (5). We have not learned the rates of PET/CT or brain MRI imaging, nor invasive mediastinal staging, and we may never learn those details or their regional variability. Though the ADAURA trial has reported a significant improvement in the rate of relapses in the central nervous system with osimertinib vs. placebo (11), the data presentation was conspicuous in not revealing the CNS relapse rates in patients who underwent a baseline brain MRI vs. head CT, despite this variable representing a critical potential confounder. To the extent that poor staging allows patients with undetected advanced disease to enroll, osimertinib must be expected to offer a profound benefit over placebo in this population that may or may not translate to an improvement in OS.
The argument may be made that a global trial like ADAURA, with variable staging and access to subsequent therapies, is an appropriate representation of “real world” practice. While we want our clinical trials to be generalizable, there is good reason to suspect that there is profound national variability in practice patterns. A lack of uniform PET/CT staging or availability of brain MRI scans is appropriately reflective of practice in some areas but not others. It would be regrettably ironic, however, to have a trial handicap the control arm due to geographically heterogeneous shortcomings, driven by practice in one part of the world where resources are very limited, only to drive a change in practice selectively in the subset of health care systems in which these limitations do not apply. “Real world” is relative, and global trials shouldn’t drive practice changes by capitalizing on the weakest links in international practice.
Adjuvant osimertinib is prone to drive a reduction in adjuvant chemotherapy
Despite the enthusiasm around adjuvant osimertinib, it has not yet demonstrated an improvement in OS, unlike adjuvant chemotherapy. Not only were patients with EGFRm+ resected NSCLC eligible to participate in the trials that established adjuvant chemotherapy as a global standard of care (these trials preceded the era of molecular testing), but patients with EGFRm+ NSCLC appear to benefit even more from adjuvant chemotherapy than patients with EGFR wild type NSCLC (12).
While ADAURA was designed to administer adjuvant osimertinib or placebo after and not necessarily instead of adjuvant chemotherapy, the fact that 40% of patients enrolled on ADAURA did not receive adjuvant chemotherapy is a concerning finding to many who consider the OS benefit with this intervention to make it a clear standard of care here. The rate of delivery of adjuvant chemotherapy on ADAURA is another key variable likely to demonstrate significant geographic variability, which will highlight that this is a factor more of cultural practice than biological limitations in the proportion of patients who are appropriate candidates. Though we do not have data to speak to this question, we should envision that many oncologists and patients alike will presume that adjuvant osimertinib will obviate the need for adjuvant chemotherapy, casting aside the treatment with a significant, proven OS benefit in favor of what is seen as a functional and perhaps superior alternative, but that has yet to demonstrate an OS benefit.
We cannot presume that even a highly significant DFS benefit will translate into an OS benefit
Strong advocates of adjuvant osimertinib point to the magnitude of the DFS benefit as an implication that this is extremely likely to predict a significant improvement in OS. While that certainly remains a possibility, we should be humbled by the pattern of DFS improvement not accompanied by OS benefit in any preceding trial of adjuvant EGFR TKI in the post-operative space (4-7), albeit with a less striking improvement in DFS in preceding studies. In the setting of advanced NSCLC, however, we have also seen a profound improvement in the secondary endpoint of progression-free survival (PFS), with a HR of 0.16, that was associated with no improvement in OS (HR, 1.19) (13).
We must acknowledge that we currently have significant gaps in our understanding of mechanisms of resistance to osimertinib, which are distinct from those of other EGFR TKIs (14). It is possible to envision a scenario in which patients assigned to adjuvant osimertinib experience a significantly superior DFS vs. duration of PFS with osimertinib in patients who receive it upon relapse, with both groups them experiencing comparable survival upon resistance to osimertinib (Figure 1A). It is quite possible that osimertinib will have a profoundly inhibitory effect on micrometastatic disease that far exceeds its effect on clinically detected, macrometastatic disease, and/or that CNS relapse or other symptomatic progression will lead to patients experiencing a clinical decline prior to administration of osimertinib that precludes them from gaining the opportunity to benefit from osimertinib and subsequent treatments. Such as scenario may lead to an improvement in OS with adjuvant osimertinib.
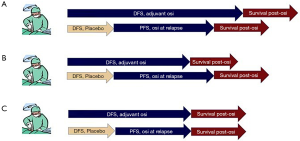
However, we must also recognize that we do not have established treatment options after patients develop progression on osimertinib, so those patients who receive adjuvant osimertinib for undetectable, asymptomatic disease post-operatively may potentially do poorly after acquired resistance and experience shorter survival than patients who do not receive adjuvant osimertinib, instead starting it in the event of relapse only after the DFS achieved on placebo, then experiencing a similar survival after acquired resistance on osimertinib (Figure 1B). This could translate to patients experiencing a superior OS by deferring osimertinib until relapse, only given to the subset that proves to need it.
Finally, patients who do not pursue adjuvant osimertinib may experience a comparable sequential duration of their DFS without osimertinib followed by the PFS achieved with osimertinib upon relapse, with both groups experiencing the same survival on treatments beyond osimertinib (Figure 1C). This would be associated with no difference in OS with adjuvant osimertinib.
The financial toxicity and other adverse effects of osimertinib
Though secondary to the potential benefit it may confer, the cost of adjuvant osimertinib is also a factor that should be considered, given its remarkably high cost and scheduled duration of 3 years (or potentially longer if physicians and/or patients are wary about discontinuing it), without a demonstrated OS benefit. These costs, along with the modest but real clinical toxicities such as typically grade 1–2 rash and diarrhea, are incurred not only for the patients otherwise destined to relapse but also for those who were already cured with surgery alone and who are now being effectively overtreated for years at a time and at great expense for no further benefit.
Some minimize this issue by noting that many of our oncology drugs are very costly, and we routinely favor osimertinib in the metastatic setting for patients with EGFRm+ NSCLC despite its cost. But for patients with advanced NSCLC being treated with other regimens in this price range, we are at least treating patients with known disease over a duration of benefit that we can measure, rather than treating patients who may or may not have any disease, for years at a time. Adjuvant osimertinib represents a distressing combination of very costly and prolonged therapy, overtreating a significant subset of this population, in the absence of a demonstrated OS benefit.
Current status and future directions
Given the current information available, it is appropriate for oncologists to test for an activating EGFR mutation in patients who would be potential candidates for adjuvant osimertinib if their tumor was found to be EGFRm+. This is especially true for patients with stage II and IIIA disease, in whom the risk of relapse is higher and the magnitude of benefit with osimertinib greatest. However, these discussions should include the caveat that adjuvant osimertinib has not demonstrated an OS benefit, and ideally this approach should not be considered a substitute for adjuvant chemotherapy.
The utility of adjuvant osimertinib would be far more established if results from the ADAURA trial demonstrate that recipients of adjuvant osimertinib subsequently demonstrates a significant OS benefit; importantly, patients on the trial remain blinded, with OS data awaited in the coming years. Whether this is achieved or not, particularly with questions about whether a potentially observed OS benefit may be spurious, there is great promise in the potential for circulating tumor DNA to help identify which patients remain at higher risk for relapse after surgery, as well as which patients on osimertinib may safely discontinue it. In addition, the ADAURA trial is just the first example of a targeted therapy for resected NSCLC in the adjuvant setting, and the coming years will also bring data on targeted therapies, including ALK and RET inhibitors as adjuvant therapy, that may help pave the way to improved outcomes here, ideally focusing on the true north of OS benefit in this setting. We have reason to hope that additional research will help us refine and ultimately transform our approach to adjuvant treatment for molecularly selected patients with early stage NSCLC.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor Grace K. Dy for the series “Evidence and Controversies in the treatment of metastatic NSCLC” published in Precision Cancer Medicine. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pcm-20-55). The series “Evidence and Controversies in the treatment of metastatic NSCLC” was commissioned by the editorial office without any funding or sponsorship. HW reports personal fees from AstraZeneca, personal fees from Genentech/Roche, outside the submitted work. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- AstraZeneca.com. ADAURA is the first global trial for an EGFR inhibitor to show statistically significant and clinically meaningful benefit in adjuvant treatment of lung cancer: plans for regulatory submission already underway. 2020. [cited 2020 September 24]. Available online: https://www.astrazeneca.com/media-centre/press-releases/2020/tagrisso-phase-iii-adaura-trial-will-be-unblinded-early-after-overwhelming-efficacy-in-the-adjuvant-treatment-of-patients-with-egfr-mutated-lung-cancer.html
- Herbst RS, Tsuboi M, John T, et al. Osimertinib as adjuvant therapy in patients (pts) with stage IB-IIIA EGFR mutation-positive (EMGFm) NSCLC after complete tumor resection: ADAURA. J Clin Oncol 2020;38:LBA5. [Crossref]
- Wu YL, Tsuboi M, He J, et al. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med 2020;383:1711-23. [Crossref] [PubMed]
- Pennell NA, Neal JW, Chaft JE, et al. SELECT: A phase II trial of adjuvant erlotinib in patients with resected epidermal growth factor receptor-mutant non-small-cell lung cancer. J Clin Oncol 2019;37:97-104. [Crossref] [PubMed]
- Kelly K, Altorki NK, Eberhardt WE, et al. Adjuvant erlotinib versus placebo in patients with stage IB-IIIA non-small-cell lung cancer (RADIANT): a randomized, double-blind, phase III trial. J Clin Oncol 2015;33:4007-14. [Crossref] [PubMed]
- Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol 2018;19:139-48. [Crossref] [PubMed]
- Wu YL, Zhong W, Wang Q, et al. CTONG1104: Adjuvant gefitinib versus chemotherapy for resected N1-N2 NSCLC with EGFR mutation: final overall survival analysis of the randomized phase III trial 1 analysis of the randomized phase III trial. J Clin Oncol 2020;38:9005. [Crossref]
- Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med 2020;382:41-50. [Crossref] [PubMed]
- Burdett S, Pignon JP, Tierney J, et al. Adjuvant chemotherapy for resected early-stage non-small cell lung cancer. Cochrane Database Syst Rev 2015;CD011430. [Crossref] [PubMed]
- Wakelee HA, Dahlberg SE, Keller SM, et al. Adjuvant chemotherapy with or without bevacizumab in patients with resected non-small-cell lung cancer (E1505): an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol 2017;18:1610-23. [Crossref] [PubMed]
- Tsuboi M, Wu Y, He J, et al. LBA1 Osimertinib adjuvant therapy in patients (pts) with resected EGFR mutated (EGFRm) NSCLC (ADAURA): central nervous system (CNS) disease recurrence. Ann Oncol 2020;31:S1177. [Crossref]
- Tsao MS, Sakurada A, Ding K, et al. Prognostic and predictive value of epidermal growth factor receptor tyrosine kinase domain mutation status and gene copy number for adjuvant chemotherapy in non-small cell lung cancer. J Thorac Oncol 2011;6:139-47. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Schoenfeld AJ, Yu HA. The evolving landscape of resistance to osimertinib. J Thorac Oncol 2020;15:18-21. [Crossref] [PubMed]
Cite this article as: West H. ADAURA: a definitive answer to the wrong question. Precis Cancer Med 2020;3:32.

