
Micrometastatic breast cancer presenting as abnormal uterine bleeding: case report
Introduction
Breast cancer (BC) is the second most common cause of cancer related deaths in the US (1). Metastatic potential is higher for invasive lobular carcinoma (ILC) than invasive ductal carcinoma (IDC) (2). Although BC frequently metastasizes to the lungs and bone, genitalia are rarely involved; and when it does, ovaries are more commonly affected than other sites (3). Even rarer is BC metastasis to the endometrium (4). In English literature, there are approximately 60 recorded cases of BC metastasis to the uterus (4-10) of which only 25 are due to IDC. Thirteen of the latter cases metastasized to the endometrium, many of which were macro-metastasis. We present a case of BC micrometastasis (<2 mm focus) to the endometrium found incidentally on an endometrial biopsy for evaluation of abnormal uterine bleeding (AUB).
We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/pcm-20-48).
Case presentation
A 46-year-old female with a history of IDC [Nottingham grade 2 (pT1c, N0, M0)] of the left breast status post nipple sparing mastectomy in 2013 and adjuvant tamoxifen therapy from 2013 to 2018, presented with a 3-week history of irregular menstrual bleeding in February 2019. Prior evaluation for AUB was in May 2016, at which time an endometrial biopsy was unremarkable. The patient was managed conservatively and had been amenorrhoeic since November 2016 (Figure 1).

The patient’s past medical history was significant for leiomyomata, endometriosis, left ovarian cyst, and stable right thyroid nodule. Family history was negative for breast or ovarian cancer. The patient was negative for BRCA1 and BRCA2 gene mutations.
Physical exam at presentation for AUB revealed unremarkable genitalia except for 10 mL of dark red-brown blood in the vagina. Bimanual exam revealed a large, anteverted uterus, irregular in contour and approximately 14–16 weeks in size. Adnexa were nontender with minimal mobility. Urine pregnancy test was negative. Pap smear was negative for intraepithelial malignancy. An endometrial biopsy revealed a predominantly proliferative endometrium and a 1.5-mm focus of atypical cells seamlessly admixed with the endometrial stroma (Figure 2A). On closer examination, the atypical cells were polygonal and larger than the surrounding stromal cells, contained abundant eosinophilic cytoplasm, enlarged irregular nuclei and variably prominent nucleoli (Figure 2B). Immunohistochemical studies highlighted that the atypical cells were positive for GATA3, E-cadherin, CK7 and mammaglobin (Figure 3) but negative for PAX8 supporting a metastatic carcinoma of mammary origin. Review of prior breast biopsy revealed that the current tumor had vague cytological resemblance to the primary tumor. Following endometrial biopsy findings, a positron emission tomography (PET) scan showed irregularly thickened and fluorodeoxyglucose (FDG) avid endometrium, but no activity elsewhere. As a result, the patient underwent a total abdominal hysterectomy (TAH) and bilateral salpingo-oophorectomy (BSO) in March 2019. The patient tolerated surgery well without any complications.
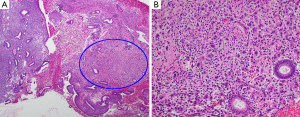
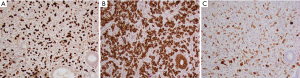
On gross examination, the uterus measured 12.0 cm × 11.0 cm × 7.0 cm and weighed 330 g. The endometrium was 0.4–1.5 cm thick and irregular. The myometrium was 2.5–4.2 cm thick with multiple circumscribed, rubbery, intramural whorled nodules 0.5–4.5 cm in greatest dimension. The entire endometrium was sampled for histologic examination which revealed a background proliferative endometrium with multiple microscopic foci of atypical cells in a somewhat desmoplastic stroma. The atypical cells were similar to those seen on the most recent endometrial biopsy. The largest tumor focus in the hysterectomy specimen measured 1.6 mm and involved an endometrial polyp. Immunohistochemical studies highlighted that the tumor cells were strongly and diffusely reactive to GATA-3, ER and mammaglobin but non-reactive to P63 and Her2. The morphology together with the immunoprofile supported a multifocal metastatic IDC to the endometrium.
The patient is currently disease free 17 months post TAH and BSO. Patient’s adherence and tolerability to surgery was assessed with post-op visits, treatment compliance, and follow-up surveillance imaging. Following TAH and BSO, the plan for chemotherapy included doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 (ddAC) given every 2 weeks for four doses followed the next day by a dose of pegfilgrastim, in addition to paclitaxel. The patient completed 4 cycles of ddAC and 3 cycles of dose dense paclitaxel regimen (175 mg/m2 every 2 weeks) and then began oral letrozole therapy. Follow-up PET CT showed no evidence of recurrent or residual disease. At her most recent follow-up (July 2020) the patient was still compliant with letrozole therapy, felt energetic, and pleased that prompt detection of her metastatic cancer facilitated early and lifesaving treatment.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013).
Discussion
To our knowledge, this is the first reported case of IDC with micrometastasis to the endometrium presenting as AUB. Although AUB was the predominant presenting symptom in prior cases of IDC metastatic to the uterus/endometrium, other symptoms included abdominal pain, abdominal mass, uterine enlargement, and urinary frequency (5-10). Nonetheless, metastatic tumor foci appeared to be larger in these cases enabling easy recognition. In our case, smaller (<2 mm) tumor foci (both on primary endometrial biopsy and subsequent hysterectomy) made it challenging to recognize the tumor cells, underscoring that micrometastasis can be easily missed. Although BC patients undergo frequent surveillance via PET or FDG to catch disease recurrence, detection of early uterine metastasis is challenging and a negative surveillance test does not conclusively rule out residual disease elsewhere. Endometrial biopsies may be the only opportunity to catch micrometastatic disease in patients with a history of BC, so when given the opportunity to examine a biopsy specimen from a patient with history of cancer, a higher level of scrutiny is warranted to increase the chances of detecting subtle micrometastasis. Consequently, a thorough histologic examination of the endometrial biopsy is warranted in BC patients with history of AUB.
This case was particularly challenging because individual tumor cells blended seamlessly with the endometrial stroma. Nonetheless, atypical cytomorphology together with the unique immunohistochemical profile of the tumor cells confirmed this to be of mammary origin. At the time of diagnosis, our patient had completed a 5-year course of adjuvant tamoxifen, which should not have increased her risk of primary endometrial cancer because she was perimenopausal (11). Although it is unclear if tamoxifen predisposes the endometrium to metastatic disease, 7/13 patients with IDC metastatic to the endometrium had a history of tamoxifen use (12-18).
A key limitation of this study is that we cannot exclude, with 100% certainty, the possibility of microscopic metastatic disease elsewhere in the body despite negative radiographic studies (PET, FDG etc.). Consequently, frequent follow-up is crucial.
Taken together, our case highlights the fact that early diagnosis of metastatic disease can be lifesaving in BC patients and that pathologists should pay careful attention to the stroma when examining endometrial biopsies from such patients. Because micrometastasis was detected early, our patient was able to undergo a timely hysterectomy.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/pcm-20-48
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pcm-20-48). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Ferlicot S, Vincent-Salomon A, Médioni J, et al. Wide metastatic spreading in infiltrating lobular carcinoma of the breast. Eur J Cancer 2004;40:336-41. [Crossref] [PubMed]
- Mazur MT, Hsueh S, Gersell DJ. Metastases to the female genital tract. Analysis of 325 cases. Cancer 1984;53:1978-84. [Crossref] [PubMed]
- Kumar NB, Hart WR. Metastases to the uterine corpus from extragenital cancers. A clinicopathologic study of 63 cases. Cancer 1982;50:2163-9. [Crossref] [PubMed]
- Razia S, Nakayama K, Tsukao M, et al. Metastasis of breast cancer to an endometrial polyp, the cervix and a leiomyoma: A case report and review of the literature. Oncol Lett 2017;14:4585-92. [Crossref] [PubMed]
- Aytekin A, Bilgetekin I, Ciltas A, et al. Lobular breast cancer metastasis to uterus during adjuvant tamoxifen treatment: A case report and review of the literature. J Cancer Res Ther 2018;14:1135-7. [Crossref] [PubMed]
- Briki R, Cherif O, Bannour B, et al. Uncommon metastases of invasive lobular breast cancer to the endometrium: a report of two cases and review of the literature. Pan Afr Med J 2018;30:268. [Crossref] [PubMed]
- Arif SH, Mohammed AA, Mohammed FR. Metastatic invasive lobular carcinoma of the breast to the endometrium presenting with abnormal uterine bleeding; Case report. Ann Med Surg (Lond) 2020;51:41-3. [Crossref] [PubMed]
- Gomez M, Whitting K, Naous R. Lobular breast carcinoma metastatic to the endometrium in a patient under tamoxifen therapy: A case report. SAGE Open Med Case Rep 2020;8:2050313X20907208.
- Akhtar A, Ratra A, Puckett Y, et al. Synchronous Uterine Metastases from Breast Cancer: Case Study and Literature Review. Cureus 2017;9:e1840. [Crossref] [PubMed]
- Committee Opinion No. 601: Tamoxifen and uterine cancer. Obstet Gynecol 2014;123:1394-7. [Crossref] [PubMed]
- Acikalin MF, Oner U, Tekin B, et al. Metastasis from breast carcinoma to a tamoxifen-related endometrial polyp. Gynecol Oncol 2005;97:946-8. [Crossref] [PubMed]
- Aydin O, Bagci P, Akyildiz EU, et al. Metastasis from breast carcinoma to endometrial polyp. Eur J Gynaecol Oncol 2008;29:666-8. [PubMed]
- Binstock A, Smith AL, Olawaiye AB. Recurrent breast carcinoma presenting as postmenopausal vaginal bleeding: A case report. Gynecol Oncol Rep 2013;10:38-40. [Crossref] [PubMed]
- Corley D, Rowe J, Curtis MT, et al. Postmenopausal bleeding from unusual endometrial polyps in women on chronic tamoxifen therapy. Obstet Gynecol 1992;79:111-6. [PubMed]
- Horn LC, Einenkel J, Baier D. Endometrial metastasis from breast cancer in a patient receiving tamoxifen therapy. Gynecol Obstet Invest 2000;50:136-8. [Crossref] [PubMed]
- Kennebeck CH, Alagoz T. Signet ring breast carcinoma metastases limited to the endometrium and cervix. Gynecol Oncol 1998;71:461-4. [Crossref] [PubMed]
- Ramalingam P, Middleton LP, Tamboli P, et al. Invasive micropapillary carcinoma of the breast metastatic to the urinary bladder and endometrium: diagnostic pitfalls and review of the literature of tumors with micropapillary features. Ann Diagn Pathol 2003;7:112-9. [Crossref] [PubMed]
Cite this article as: Gomez N, McNeely M, Asirvatham JR, Akki AS. Micrometastatic breast cancer presenting as abnormal uterine bleeding: case report. Precis Cancer Med 2020;3:27.

