
Circulating tumor cells: moving forward into clinical applications
Introduction
Cancer, as a devastating disease, is one of the leading causes of death around the world (1). It is more likely to be curable at early stages, therefore its early detection can increase the chances of successful treatment, resulting in a reduction of cancer-related deaths. Moreover, cancer monitoring is also crucial for the real-time clinical follow-up and evaluation of minimal residual disease (MRD) (2).
Regarding difficulties to obtain tissue samples, liquid biopsy, especially blood-based bio-resources including circulating tumor cells (CTCs) (3), circulating tumor DNA (ctDNA) (4), extracellular vesicles (EVs) (5) and tumor-educated platelets (TEPs) (6), provides an alternative minimally invasive method for cancer detection and real-time follow-ups of patients (7,8).
One of the most studied bio-sources so far are CTCs. These cancer cells have been shed into the blood and/or lymphatic circulation from primary and/or metastatic site(s) which might result in the formation of (micro)metastases in a new distant micro-environment (7). Due to their remarkable potential, the analysis of CTCs in cancer patients has recently received widespread attention for clinical implications.
CTCs can reflect tumor mutations and heterogeneity in different stages of malignancy as a snapshot of the cancer progression at a given time (9). The presence of CTCs might predict the presence of metastasis before clinical or radiographical signs, and possibly enabling the early detection of cancer initiation or recurrence (2).
In this review, we discussed about important clinical trials in different cancer types and how these studies can guide clinician for precision medicine.
Current clinical applications for CTCs
A higher number of CTCs is associated with poorer prognosis (10). Similarly, despite of treatment, the present of CTCs can be predictor of shorter overall survival (OS). Hence, the progression of the disease and therapeutic response/resistance can be monitored by enumerating and characterizing CTCs.
It is notably to say that, although CTC enumeration is possible, their low number in circulation as well as their molecular heterogeneity makes isolation and characterization more challenging, and therefore they are not yet used comprehensively as a routine clinical test. To demonstrate the clinical relevance of CTCs, several trial studies have been performed or are still ongoing to finally confirm their real potential to fully transfer this biomarker into clinical decision making.
For the CTC detection in cancer patients, the only method with a proven clinical validity (11) and approval of the Food & Drugs Agency (FDA) is the CellSearch® system (Menarini Silicon Biosystems, Inc; Bologna, Italy). This method allows capture and detection of CTCs based on the expression of epithelial markers (e.g., EpCAM, PanCK). Briefly, magnetic beads attached to antibodies against EpCAM are used for the enrichment step; then, CTCs can be visualized by fluorescent stains against cytokeratins and nucleic acids, allowing to distinguish them from leukocytes that are CD45(+) (12).
CTCs detected with this technology have clinical validity in prognosis of breast cancer (13), colorectal cancer (CRC) (14), prostate cancer (15), small cell lung cancer (SCLC) (16), non-small cell lung cancer (NSCLC) (17) and pancreatic adenocarcinoma (18). The prognostic role of CTCs has been demonstrated in many other cancer types (10,19,20), and we will describe some of the most important clinical insights for the aforementioned malignancies (Figure 1).
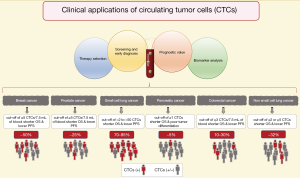
In metastatic breast cancer (MBC), CTCs can be detected in up to 50% of patients (10). Bidard et al. performed a pooled analysis of 1,944 from 17 different centers proving that ≥5 CTCs per 7.5 mL of blood are associated with a shorter OS and a lower progression-free survival (PFS), thus establishing clinical validity of CTC enumeration (11,13), and it has been shown that this is independent to the presence of distant tumor cells in the bone marrow (21). Correspondingly, the use of CTCs for staging in breast cancer designed cM0(i+) is already included in the 4th edition of the WHO Classification of Tumours of the Breast (22) and in the 8th edition of the American Joint Committee on Cancer (AJCC) cancer staging manual (23). Moreover, Cristofanilli et al. have proposed to further include a sub-classification for MBC stage IV into “indolent” and “aggressive” accordingly to the cut-off of ≥5 CTCs per 7.5 mL of blood; in a pooled analysis of 18 cohorts, they demonstrated that the CTC enumeration is independent to clinical and molecular variants (24). Beside the role of CTCs in staging and prognosis in breast cancer, several randomized prospective clinical trials have been run during the last decade in order to prove the clinical utility in the management of MBC with contrasting results (19). Smerage et al. in a interventional clinical trial (SWOG S0500; NCT00382018) failed to prove clinical utility in the early change of chemotherapy regime in patients with persistent high number of CTCs (≥5 CTCs in 7.5 mL of blood) after 21 days from the first-line chemotherapy, although the prognostic role of CTCs enumeration was clearly supported (25). However, it is important to notice that no efficient treatment could be given to these MBC patients, thus whatever the biomarker used, in that case, the data would have been the same: ‘no clinical relevance’. Recently, the STIC-METABREAST trial (NCT01710605) is ongoing to demonstrate that in MBC with HER2-negative and hormone receptor-positive, the use of CTCs is clinically relevant to a clinical-drive decision for the selection of chemotherapy (≥5 CTCs in 7.5 mL of blood) or endocrine therapy (<5 CTCs in 7.5 mL of blood); this interventional clinical trial should demonstrate the clinical utility of CTCs (26). However, the standard treatment care for these MBC has changed during the time of the trial due to the introduction of CDK4/6 inhibitors (27).
In metastatic CRC (mCRC) the association of a higher number of CTCs is related with poorer prognosis (14). The CTC counts vary greatly between studies, with ~10% to 30% of CTC-positive patients at baseline before treatment (14). It has been proposed that this low amount of CTCs, compared for example to breast or prostate cancer, is the result of the filtration function of the liver (28). A cut-off of ≥3 CTCs per 7.5 mL of blood has been demonstrated to be a good predictor for OS and PFS (29,30); subsequently other studies have used a cut-off of even ≥1 CTC per 7.5 mL of blood with the same purpose (14,31). Currently, there is not a well-defined cut-off, therefore no clinical utility has been demonstrated in mCRC neither.
In SCLC, between 70–95% of patients have detectable CTCs (16). Moreover, the number of CTCs is significantly higher compared with other cancer types, this supports the use of CTC detection methods in SCLC. A multi-center prospective study showed that the existence of low number of CTCs can predict the stage of SCLC in localized or extended disease (32). Furthermore, the presence of higher number of CTCs after the first cycle of chemotherapy is associated with a worse OS and PFS (10,32). The cut-off for this cancer type is highly variable between studies (from >2 to >50) (10,16).
In contrast to SCLC, NSCLC presents fewer number of CTCs and in fewer number of patients. Krebs et al. reported that only 32% of patients with metastatic disease presented ≥2 CTCs per 7.5 mL of blood (33). Even though, it has been possible to prove the association with the worst OS and PFS in patients with ≥2 or ≥5 CTCs (17). The cut-off of ≥5 CTCs at baseline correlates with the observation of computer tomography (CT) scan response to treatment (34); and also is related with an unfavorable response to tyrosine kinase inhibitors (35).
Similarly, in pancreatic adenocarcinoma the detection rate of CTCs by using the CellSearch® system is rather low. The rate of patients with capture of CTCs (cut-off of ≥1 CTCs) has been reported to be as low as 5% (18), however, this subgroup of patients was associated with shorter OS and poor tumor differentiation (18).
In prostate cancer, CTCs have been evaluated in localized and metastatic status. In localized prostatic cancer, the detection of CTCs might be hampered by their low number in blood (36). However, Kuske et al. reported the detection of CTCs after radical prostatectomy by using three different methods, suggesting their enumeration as a marker of MRD (37). Still few studies have addressed CTCs in localized prostatic cancer (36), but the new clinical trial run in the European PROLIPSY project will answer whether we can use the liquid biopsy to diagnose prostate cancer and then to evaluate its aggressivity. Meanwhile, in metastatic prostate cancer, a cut-off of ≥5 CTCs per 7.5 mL of blood has been used by several clinical trials, demonstrating clinical validity due to its association with shorter OS (36). Moreover, Scher et al. demonstrated that CTC enumeration outperforms to the prostatic specific antigen (PSA) as an early treatment response marker (15,36) and it has been suggested their use as a surrogate endpoint in clinical trials for OS (38). At this time, a clinical trial is going on to demonstrated clinical utility for the enumeration of CTCs in metastatic castration resistance prostate cancer (TACTIK study; NCT03101046), in which the cut-off of ≥5 CTCs per 7.5 mL is used as marker to establish resistance to docetaxel, as an indication to change the chemotherapy regimen. Moreover, the detection of the androgen receptor variant 7 (AR-V7) in CTCs has shown clinical utility as prognosis and prediction to endocrine therapy response. Armstrong et al. evaluated the detection of AR-V7 by two different methods based in mRNA detection in CTCs, in a multicenter prospective clinical trial (PROPHECY study; NCT02269982), showing that the positivity to the AR-V7 variant in CTCs is associated with shorter OS and PFS when patients are under an anti-androgen therapy regime, suggesting that those patients might benefit from a change in their therapeutic approach (39). Additionally, techniques allowing the visualization of intact CTCs can provide relevant cytological information that so far other methods cannot; for instance Scher et al. reported that only the nuclear localization of the AR-V7 variant is relevant for the therapeutic guidance (40-42).
The detection of CTCs is not only limited to the aforementioned malignances. Indeed, one recent example is the CIRCUTEC study (NCT02119559) in which, by means of three different technologies, Garrel et al. demonstrated the association of the number of CTCs in blood with an early response to treatment in head and neck squamous cell carcinoma (20). Currently, more clinical trials are active in order to identify new therapeutic context in different cancer types.
Future applications and technologies in the CTC field
Currently, there are many efforts done to demonstrate the clinical relevance of CTCs in addition of their enumeration; some examples are (I) the detection of PD-L1 in CTCs to predict response to the immune checkpoint inhibitor therapy (e.g., nivolumab), for instances in breast cancer, head and neck squamous cell carcinoma or NSCLC, in which the frequency of PD-L1 expression can be evaluated by immune and molecular approaches (43-45), (II) similarly, the detection of other biomarkers and mutations (e.g., estrogen receptor, HER2 and KRAS status) that can be targeted by therapeutic agents (2,19). Moreover, the enumeration and analysis of clusters of CTCs and its “circulating micro-environment” in the bloodstream have been associated with prognosis outcomes in patients; and specific epigenetic changes are associated with these clusters (46-48). Another promising use of CTCs is in the context of MRD where the number of CTCs after curative intent treatments can be used as a marker of recurrence (2). Nevertheless, at the moment of writing this work, none of these approaches have been fully incorporated into the clinical practice outside of translational clinical trials.
On the other hand, other innovative methods for the enrichment, capture and enumeration of CTCs have been developed. One example is the Parsortix™ PC1 system (ANGLE North America, Inc., King of Prussia, PA, USA), a microfluid device that captures CTCs based on size and deformability, this method have the theoretical advantage of capture CTCs with lower expression of epithelial markers (49); in order to demonstrate the validity of this method in MBC, currently a clinical trial (ANG-002; NCT03427450) is ongoing with this aim, as well as to demonstrated its utility in different types of evaluations. Other methods include those that are based on: different markers specific of epithelial cells, photoacoustic, electric charges, metabolisms, and their functionality, however none of these methods are fully validated for clinical use (50-53). In addition, there are different approaches for the technical equipment to study rare CTCs surrounded by normal hematopoietic cells: from microfluid chips devices (50,54,55) to leukapheresis systems (56) where a high volume of blood is screened, these methods that can also be based in surface markers and/or biomechanical properties independently, will provide a wider approach to capture CTCs in early stages or to analyze the CTC-clusters (57,58). Another aspect in development is the analysis of single cell CTCs, these methods of isolation can offer different degrees of information about the genome, transcriptome, proteome and secretome level, therefore expanding the clinical applications for CTCs (2).
Conclusions
CTCs as real-time liquid biopsy represents a promising approach for personalized treatment in oncology. The generalized use of CTCs in the daily clinical practice is not yet a reality, and it has been hampered in the past by their small number in the circulation, and their phenotypic changes during cancer progression. Nonetheless, their enumeration with robust and reproducible technologies provides valuable information in specific clinical settings and it is just a question of time to fully identify more clinical scenarios in which CTCs can guide therapeutics and clinical managements in clinical practice. Moreover, the current and very active development of alternatives to enrich, detect and characterize CTCs is creating a more comprehensive field in which the genome, transcriptome, proteome and secretome of CTCs can be analyzed and provide relevant information of prognosis, status and prediction to therapies. Thus, projects, such as the European CANCER-ID, European Liquid Biopsy Academy (ELBA), European Liquid Biopsy Society (ELBS) networks or the USA based BloodPAC, have been initiated to meet these challenges.
Acknowledgments
Funding: This work was supported by (I) ELBA project received funding from the European Union Horizon 2020 Research and Innovation program under the Marie Skłodowska-Curie grant agreement No 765492, (II) CANCER-ID, an Innovative Medicines Initiative Joint Undertaking under grant agreement no. 115749, resources of which are from the European Union’s Seventh Framework Program (FP7/2007-2013) (www.cancer-id.eu) and EFPIA companies’ in-kind contribution, (III) the National Institute of Cancer (INCa, http://www.e-cancer.fr).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pcm.2019.11.07). This project has received funding from the European Union Horizon 2020 Research and Innovation program under the Marie Skłodowska-Curie grant agreement No 765492. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Pantel K, Alix-Panabières C. Liquid biopsy and minimal residual disease - latest advances and implications for cure. Nat Rev Clin Oncol 2019;16:409-24. [Crossref] [PubMed]
- Alix-Panabières C, Pantel K. Circulating tumor cells: liquid biopsy of cancer. Clin Chem 2013;59:110-8. [Crossref] [PubMed]
- Heitzer E, Ulz P, Geigl JB. Circulating tumor DNA as a liquid biopsy for cancer. Clin Chem 2015;61:112-23. [Crossref] [PubMed]
- Sheridan C. Exosome cancer diagnostic reaches market. Nat Biotechnol 2016;34:359-60. [Crossref] [PubMed]
- Best MG, Sol N, Kooi I, et al. RNA-seq of tumor-educated platelets enables blood-based pan-cancer, multiclass, and molecular pathway cancer diagnostics. Cancer Cell 2015;28:666-76. [Crossref] [PubMed]
- Pantel K, Alix-Panabières C. Circulating tumour cells in cancer patients: challenges and perspectives. Trends Mol Med 2010;16:398-406. [Crossref] [PubMed]
- Alix-Panabières C, Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov 2016;6:479-91. [Crossref] [PubMed]
- Brown HK, Tellez-Gabriel M, Cartron PF, et al. Characterization of circulating tumor cells as a reflection of the tumor heterogeneity: myth or reality? Drug Discov Today 2019;24:763-72. [Crossref] [PubMed]
- Riethdorf S, O’Flaherty L, Hille C, et al. Clinical applications of the CellSearch platform in cancer patients. Adv Drug Deliv Rev ;2018125:102-21. [PubMed]
- Bidard FC, Peeters DJ, Fehm T, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol 2014;15:406-14. [Crossref] [PubMed]
- Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 2004;10:6897-904. [Crossref] [PubMed]
- Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004;351:781-91. [Crossref] [PubMed]
- Huang X, Gao P, Song Y, et al. Meta-analysis of the prognostic value of circulating tumor cells detected with the CellSearch System in colorectal cancer. BMC Cancer 2015;15:202. [Crossref] [PubMed]
- Scher HI, Jia X, de Bono JS, et al. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: a reanalysis of IMMC38 trial data. Lancet Oncol 2009;10:233-9. [Crossref] [PubMed]
- Foy V, Fernandez-Gutierrez F, Faivre-Finn C, et al. The clinical utility of circulating tumour cells in patients with small cell lung cancer. Transl Lung Cancer Res 2017;6:409-17. [Crossref] [PubMed]
- Lindsay CR, Blackhall FH, Carmel A, et al. EPAC-lung: pooled analysis of circulating tumour cells in advanced non-small cell lung cancer. Eur J Cancer 2019;117:60-8. [Crossref] [PubMed]
- Bidard FC, Huguet F, Louvet C, et al. Circulating tumor cells in locally advanced pancreatic adenocarcinoma: the ancillary CirCe 07 study to the LAP 07 trial. Ann Oncol 2013;24:2057-61. [Crossref] [PubMed]
- Cabel L, Proudhon C, Gortais H, et al. Circulating tumor cells: clinical validity and utility. Int J Clin Oncol 2017;22:421-30. [Crossref] [PubMed]
- Garrel R, Mazel M, Perriard F, et al. Circulating tumor cells as a prognostic factor in recurrent or metastatic head and neck squamous cell carcinoma: the CIRCUTEC prospective study. Clin Chem 2019;65:1267-75. [Crossref] [PubMed]
- Bidard FC, Vincent-Salomon A, Sigal-Zafrani B, et al. Prognosis of women with stage IV breast cancer depends on detection of circulating tumor cells rather than disseminated tumor cells. Ann Oncol 2008;19:496-500. [Crossref] [PubMed]
- Lakhani S, Ellis I, Schnitt S, et al. WHO classification of tumours of the breast. 4th ed. Lyon: IARC Press, 2012.
- Amin MB, Edge S, Greene F, et al. AJCC Cancer Staging Manual. 8th ed. New York: Springer Publishing, 2017.
- Cristofanilli M, Pierga JY, Reuben J, et al. The clinical use of circulating tumor cells (CTCs) enumeration for staging of metastatic breast cancer (MBC): international expert consensus paper. Crit Rev Oncol Hematol 2019;134:39-45. [Crossref] [PubMed]
- Smerage JB, Barlow WE, Hortobagyi GN, et al. Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J Clin Oncol 2014;32:3483-9. [Crossref] [PubMed]
- Bidard FC, Jacot W, Dureau S, et al. Abstract GS3-07: Clinical utility of circulating tumor cell count as a tool to chose between first line hormone therapy and chemotherapy for ER+ HER2-metastatic breast cancer: results of the phase III STIC CTC trial. San Antonio: 2018 San Antonio Breast Cancer Symposium, 2019. doi:
10.1158/1538-7445.SABCS18-GS3-07 . - Shah M, Nunes MR, Stearns V. CDK4/6 inhibitors: game changers in the management of hormone receptor-positive advanced breast cancer? Oncology 2018;32:216-22. [PubMed]
- Denève E, Riethdorf S, Ramos J, et al. Capture of viable circulating tumor cells in the liver of colorectal cancer patients. Clin Chem 2013;59:1384-92. [Crossref] [PubMed]
- Burz C, Pop VV, Buiga R, et al. Circulating tumor cells in clinical research and monitoring patients with colorectal cancer. Oncotarget 2018;9:24561-71. [Crossref] [PubMed]
- Cohen SJ, Punt CJ, Iannotti N, et al. Prognostic significance of circulating tumor cells in patients with metastatic colorectal cancer. Ann Oncol 2009;20:1223-9. [Crossref] [PubMed]
- Seeberg LT, Waage A, Brunborg C, et al. Circulating tumor cells in patients with colorectal liver metastasis predict impaired survival. Ann Surg 2015;261:164-71. [Crossref] [PubMed]
- Hiltermann TJ, Pore MM, van den Berg A, et al. Circulating tumor cells in small-cell lung cancer: a predictive and prognostic factor. Ann Oncol 2012;23:2937-42. [Crossref]
- Krebs MG, Sloane R, Priest L, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol 2011;29:1556-63. [Crossref] [PubMed]
- Punnoose EA, Atwal S, Liu W, et al. Evaluation of circulating tumor cells and circulating tumor DNA in non-small cell lung cancer: association with clinical endpoints in a phase II clinical trial of pertuzumab and erlotinib. Clin Cancer Res 2012;18:2391-401. [Crossref] [PubMed]
- Yang B, Qin A, Zhang K, et al. Circulating tumor cells predict prognosis following tyrosine kinase inhibitor treatment in EGFR-mutant non-small cell lung cancer patients. Oncol Res 2017;25:1601-6. [Crossref] [PubMed]
- Maas M, Hegemann M, Rausch S, et al. Circulating tumor cells and their role in prostate cancer. Asian J Androl 2017; [Epub ahead of print]. [PubMed]
- Kuske A, Gorges TM, Tennstedt P, et al. Improved detection of circulating tumor cells in non-metastatic high-risk prostate cancer patients. Sci Rep 2016;6:39736. [Crossref] [PubMed]
- Heller G, Fizazi K, McCormack R, et al. The added value of circulating tumor cell enumeration to standard markers in assessing prognosis in a metastatic castration-resistant prostate cancer population. Clin Cancer Res 2017;23:1967-73. [Crossref] [PubMed]
- Armstrong AJ, Halabi S, Luo J, et al. Prospective multicenter validation of androgen receptor splice variant 7 and hormone therapy resistance in high-risk castration-resistant prostate cancer: the PROPHECY study. J Clin Oncol 2019;37:1120-9. [Crossref] [PubMed]
- Scher HI, Lu D, Schreiber NA, et al. Association of AR-V7 on circulating tumor cells as a treatment-specific biomarker with outcomes and survival in castration-resistant prostate cancer. JAMA Oncol 2016;2:1441-9. [Crossref] [PubMed]
- Pantel K, Hille C, Scher HI. Circulating tumor cells in prostate cancer: from discovery to clinical utility. Clin Chem 2019;65:87-99. [Crossref] [PubMed]
- Scher HI, Graf RP, Schreiber NA, et al. Nuclear-specific AR-V7 protein localization is necessary to guide treatment selection in metastatic castration-resistant prostate cancer. Eur Urol 2017;71:874-82. [Crossref] [PubMed]
- Mazel M, Jacot W, Pantel K, et al. Frequent expression of PD-L1 on circulating breast cancer cells. Mol Oncol 2015;9:1773-82. [Crossref] [PubMed]
- Strati A, Koutsodontis G, Papaxoinis G, et al. Prognostic significance of PD-L1 expression on circulating tumor cells in patients with head and neck squamous cell carcinoma. Ann Oncol 2017;28:1923-33. [Crossref] [PubMed]
- Guibert N, Delaunay M, Lusque A, et al. PD-L1 expression in circulating tumor cells of advanced non-small cell lung cancer patients treated with nivolumab. Lung Cancer 2018;120:108-12. [Crossref] [PubMed]
- Balakrishnan A, Koppaka D, Anand A, et al. Circulating tumor cell cluster phenotype allows monitoring response to treatment and predicts survival. Sci Rep 2019;9:7933. [Crossref] [PubMed]
- Szczerba BM, Castro-Giner F, Vetter M, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 2019;566:553-7. [Crossref] [PubMed]
- Gkountela S, Castro-Giner F, Szczerba BM, et al. Circulating tumor cell clustering shapes DNA methylation to enable metastasis seeding. Cell 2019;176:98-112.e14. [Crossref] [PubMed]
- Miller MC, Robinson PS, Wagner C, et al. The ParsortixTM cell separation system-A versatile liquid biopsy platform. Cytometry A 2018;93:1234-9. [Crossref] [PubMed]
- Tang Y, Wang Z, Li Z, et al. High-throughput screening of rare metabolically active tumor cells in pleural effusion and peripheral blood of lung cancer patients. Proc Natl Acad Sci U S A 2017;114:2544-9. [Crossref] [PubMed]
- Alix-Panabières C, Pantel K. Challenges in circulating tumour cell research. Nat Rev Cancer 2014;14:623-31. [Crossref] [PubMed]
- Galanzha EI, Menyaev YA, Yadem AC, et al. In vivo liquid biopsy using Cytophone platform for photoacoustic detection of circulating tumor cells in patients with melanoma. Sci Transl Med 2019; [Crossref] [PubMed]
- Alix-Panabières C. EPISPOT assay: detection of viable DTCs/CTCs in solid tumor patients. Recent Results Cancer Res 2012;195:69-76. [Crossref] [PubMed]
- Cho H, Kim J, Song H, et al. Microfluidic technologies for circulating tumor cell isolation. Analyst 2018;143:2936-70. [Crossref] [PubMed]
- Sequist LV, Nagrath S, Toner M, et al. The CTC-chip: an exciting new tool to detect circulating tumor cells in lung cancer patients. J Thorac Oncol 2009;4:281-3. [Crossref] [PubMed]
- Kim TH, Wang Y, Oliver CR, et al. A temporary indwelling intravascular aphaeretic system for in vivo enrichment of circulating tumor cells. Nat Commun 2019;10:1478. [Crossref] [PubMed]
- Castro J, Sanchez L, Nuñez MT, et al. Screening circulating tumor cells as a noninvasive cancer test in 3388 individuals from high-risk groups (ICELLATE2). Dis Markers 2018;2018:4653109. [Crossref] [PubMed]
- Aceto N, Bardia A, Miyamoto DT, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014;158:1110-22. [Crossref] [PubMed]
Cite this article as: Eslami-S Z, Cortés-Hernández LE, Alix-Panabières C. Circulating tumor cells: moving forward into clinical applications. Precis Cancer Med 2020;3:4.

