
Targeting ErbB2 aberrations in non-small cell lung cancer
Introduction
Human epidermal growth factor 2 (HER2 or ErbB2) is a receptor tyrosine kinase of the ERBB family, which also includes EGFR, HER3 and HER4. These receptors each contain an extracellular ligand binding region, a hydrophobic transmembrane domain as well as an intracellular domain with tyrosine kinase catalytic activity. When ligands bind to the extracellular domains of EGFR, HER3 and HER4, these receptors form heterodimers, which in turn activate various signaling cascades including the raf/mitogen-activated protein kinase (MAPK), phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K), and protein kinase C (PKC) pathways. These pathways play essential roles in cell proliferation, differentiation, angiogenesis and protection from apoptosis. Interestingly, although it is structurally quite similar to its relatives, there are no identified ErbB2-specific ligands. Instead, ErbB2 is constitutively activated, serves as the preferred dimerization partner for the other receptor kinases and heterodimers containing ErbB2 are rather stable and potent (1). As a result, deregulation and overexpression of ErbB2 leads to increased formation of these heterodimers, which in turn activate signaling cascades that potentiate cell proliferation, and ultimately serves as a potent driver of oncogenesis. Therefore, two main strategies have emerged in targeting ErbB2 amplification and mutations: antibodies (and antibody-drug conjugates), which are directed at the extracellular domain, and tyrosine kinase inhibitors (TKIs), which target the intracellular kinase domain. Especially promising among these is poziotinib, a novel, quinazoline-based irreversible TKI, which has shown efficacy in patients with exon 20 mutations.
Case presentation
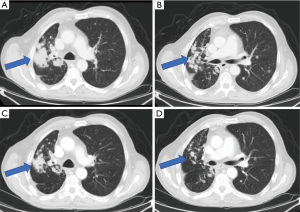
A 68-year-old gentleman with a remote smoking history and no significant medical comorbidities presented initially with a persistent cough that had worsened over the course of several months. A chest computed tomography (CT) showed a right upper lobe mass with associated adenopathy, and a positron emission tomography (PET)/CT demonstrated PET-avid liver, adrenal and bony metastases. He was referred for a CT-guided biopsy of the right iliac bone, and results showed a TTF1+ adenocarcinoma compatible with lung origin. Unfortunately, the specimen was not sufficient for further molecular testing. Further workup included a brain magnetic resonance imaging (MRI), which showed multiple small (less than 4 mm) lesions, consistent with brain metastases, and a repeat bone biopsy, which ruled out the presence of EGFR/ALK abnormalities and showed advanced, ErbB2 YVMA mutated lung adenocarcinoma. The patient received stereotactic radiosurgery (SRS) for the brain metastases, and a repeat brain MRI showed resolution of all but one lesion, which showed treatment-related changes. He was started on treatment with carboplatin/pemetrexed/pembrolizumab and initially had an excellent response. However, after approximately 8–9 months of treatment, he was found to have bony progression in the left hemipelvis. Extended molecular testing at that time confirmed the above ErbB2 mutation. He was then given second-line treatment with docetaxel/ramucirumab. The patient developed considerable adverse effects related to this regimen after just 2–3 cycles and was subsequently started on the anti-ErbB2 drug conjugate, ado-trastuzumab emtansine (T-DM1) at an outside institution. However, within several months, he was found to have progression in central nervous system (CNS) metastases and received whole brain radiation therapy. He then resumed care at our institution and was started on poziotinib 16mg daily on a research protocol. After just one cycle of treatment, imaging showed improvement with significant reduction in burden of metastatic disease (Figure 1). Unfortunately, later significant toxicities developed preventing patient from being able to continue study medicine with subsequent decline in performance status.
Molecular tumor board
Oncogenic activation of ErbB2 occurs primarily by three mechanisms: amplification and overexpression, somatic mutations of the receptor and inhibition of phosphatase activity (2). ErbB2 amplification was first discovered in breast cancer and is found in 15–30% of invasive breast carcinomas. In that setting, it serves both prognostic and predictive value as it is associated with shorter progression-free and overall survival (3). ErbB2 amplification has now been identified in various other cancers as well, including ovarian, uterine, gastric, colon, bladder, lung and is a validated treatment target in gastric adenocarcinoma. More recent studies have shown that deregulation of the ErbB2 gene can also result from various somatic mutations. First identified in lung adenocarcinoma, these have now been identified in various other malignancies as well. As with EGFR mutations, which are mutually exclusive with ErbB2 mutations, the latter have more commonly been identified in non-smokers, women, Asians and in adenocarcinoma (4). Activation of ErbB2 can result from missense mutations or insertions in the kinase domain, as well as from missense mutations (such as the S310 single-site substitution) or deletions of the extracellular domain. The most common alterations of ErbB2 involve in-frame insertions in exon 20, such as the YVMA insertion at codon 776. These mutations result in a conformational change in the autoinhibitory αC-β4 loop, which narrows the ATP-binding site and as a result promotes constitutive kinase activity (5). Less common are missense mutations in the extracellular domain. These promote constitutive kinase activity by either inducing disulfide bond formation (such as S335C or G309E) or by increasing C-terminal tail phosphorylation (such as S3105 and S310Y) (6). Lastly, ErbB2 can be activated by inhibition of phosphatase activity, and this is supported by in vitro studies which have shown that inactivation of the PTPN12 phosphatase promotes ErbB2 activation (7).
ErbB2 alterations can be identified using various diagnostic assays including enzyme-linked immunosorbent assay (ELISA), immunohistochemistry (IHC), fluorescence in situ hybridization (FISH), as well as next generation sequencing (NGS) (4,8,9). However, no gold standard exists. Traditionally, the most commonly used methods to detect ErbB2 amplifications have been IHC, to quantify the ErbB2 protein as well as FISH to determine the ErbB2 gene copy number. While the former is widely available and easy to perform, results are variable and depend on a variety of factors including the antibody used as well the cutoffs used to define positivity. FISH, on the other hand, is not as easily available but much more specific. As a result, in breast cancer, IHC is often used a screening test, and a reflex FISH is performed if results are equivocal. In non-small cell lung cancer (NSCLC), however, this approach has not been favored as there is often significant discordance between IHC and FISH results (10). More recently, dual in situ hybridization (DISH) has been shown to be better at detection of ErbB2 amplification compared to FISH (11). ErbB2 mutations, on the other hand, can be detected using Sanger sequencing, NGS as well as mass spectrometry genotyping. Of these, NGS is highly sensitive, and allows for the detection of various mutations, rearrangements as well as amplifications, and is the preferred methodology for molecular testing in lung cancer as it can detect all potential actionable alterations.
Patients with ErbB2 aberrations have a shorter overall survival than those with other oncogenic drivers (12), and there remains a significant unmet need to develop the optimal targeted therapy for these patients. At present, two main strategies have been used in targeting ErbB2 amplification and mutations: antibodies (and antibody-drug conjugates), which are directed at the extracellular domain, and TKIs, which target the intracellular kinase domain (2).
Trastuzumab, a humanized monoclonal antibody that targets the extracellular domain of the ErbB2 receptor, is standard of care in the management of ErbB2-amplified breast cancer. Unfortunately, early studies did not show similar efficacy or survival benefit in NSCLC as a single agent (13) or when combined with gemcitabine-cisplatin (14) or docetaxel (15). However, it is important to note that these early trials selected patients on the basis of ErbB2 positivity on IHC and we now know that ErbB2 mutations, which do not always result in ErbB2 overexpression, likely play a more important role in oncogenesis in NSCLC. More recent retrospective analyses have provided more promising results. A 2013 study identified 65 patients with ErbB2 mutations, 16 of which were treated with trastuzumab-based therapies after conventional chemotherapy, and reported a disease control rate of 96% (16). A similar retrospective analysis of a cohort of 101 patients with ErbB2 exon 20 mutations identified 65 patients who had received ErbB2-directed therapy after conventional chemotherapy. Of these, 57 patients had received trastuzumab in combination with chemotherapy, suggesting a disease control rate of 50%, and supporting the use of combined regimens (17). T-DM1, an ErbB2-targeted antibody-drug conjugate, has shown benefit as well. A study by Li et al. in 2018 showed a partial response rate of 44% and median progression-free survival (PFS) of 5 months in 18 patients with advanced, ErbB2-mutant lung adenocarcinomas who were treated with T-DM1 (18). These patients had various ErbB2 mutations including exon 20 insertions and point mutations in the transmembrane and extracellular domains.
TKIs have been studied in this setting as well. Afatinib, an irreversible inhibitor of EGFR, ErbB2, and HER4, has shown some efficacy in case reports and retrospective reviews. In a series of three patients with various ErbB2 exon 20 mutations, each achieved a partial response, lasting as long as 4 months (19). Another case report showed a more durable, 10-month response in a patient with the common exon 20 YVMA insertion (20), and an international retrospective review of 27 patients with metastatic ErbB2-mutant disease showed a partial response rate of 15% (21). Dacomitinib, a second-generation, irreversible inhibitor of EGFR, ErbB2, and HER4, has been studied as well. A phase two trial enrolled 30 patients with ErbB2 mutations or amplifications and found a partial response rate of 12% that lasted as long as 14 months in patients who had ErbB2-mutated disease. No partial responses occurred in those with ErbB2 amplification or in those with the most common exon 20 insertion, A775_G776insYVMA (22). Neratinib, another second-generation TKI, produced a response rate of 19% and progression free survival of up to 18-plus months in a cohort of 43 patients with ErbB2 mutations, when it was used in combination with the mammalian target of rapamycin (mTOR) inhibitor temsirolimus (23). Lapatinib, a dual TKI which inhibits ErbB1 and ErbB2, has been shown to be effective in HER2-positive breast cancer but has not demonstrated activity in NSCLC. More recently, preclinical data suggests that osimertinib may be effective in targeting ErbB2 aberrations as well, particularly amplification (24). Tarloxotinib, a prodrug that releases an irreversible EGFR/ErbB2 TKI under hypoxic conditions, has also shown promising results in a recent in vivo study of murine xenografts of two adenocarcinoma cell lines with exon 20 mutations (25,26). After 4 weeks of treatment with tarloxotinib, the authors found significant reduction in tumor burden as compared to afatinib which did not alter tumor growth.
Although TKIs have certainly shown some activity, response rates are low and there are no approved agents for ErbB2-mutant lung cancer. Newer data is encouraging. TAK-788 (AP32788), an investigational TKI that targets EGFR and ErbB2 mutations, is being studied in a phase I/II, open-label, multicenter study which administered the drug in dose escalation and expansion cohorts, which were based on tumor genotype. Initial data showed a partial response rate of 21% (n=14) in patients with EGFR exon 20 insertions (27) and more recently published data shows an objective response rate of 54% and disease control rate of 89% in the 26 patients assessed (28). The ongoing clinical trial will help assess efficacy in patients with ErbB2 alterations (29). Equally exciting is data on poziotinib, a novel, quinazoline-based irreversible inhibitor of EGFR, ErB2 and HER4. This potent, small and flexible inhibitor is hypothesized to be more effective in those with exon 20 mutations as these alterations reduce the size of the drug-binding pocket, rendering most larger TKIs ineffective (30). A phase I/II trial of 50 patients with metastatic, EGFR or ErbB2 exon 20-mutated lung adenocarcinoma showed an objective response rate of 50% and disease control rate of 83% in the latter group (31). The mean PFS for these patients was 5.1 months (Table 1). Further studies are needed to determine the efficacy of these various agents.
Table 1
| Agents | Mechanism of action | Eligibility | Efficacy | AEs | Reference |
|---|---|---|---|---|---|
| T-DM1 | Cytotoxic anti-microtubule agent released within the target cells upon degradation of the human HER2-T-DM1 complex in lysosomes | IHC 3+ | 44% (partial response rate) | Fatigue, nausea, bone and joint pain, muscle pain, thrombocytopenia headache, constipation, nerve damage, anemia, hypo-potassium | (18) |
| Mutation | |||||
| Amplified/mutation | |||||
| Pertuzumab, trastuzumab | Humanized mAb directed against the extracellular domain of the tyrosine kinase receptor HER2 | Amplified | 50–96% (disease control rate) | Cardiac failure, cardiomyopathy, CHF, pulmonary infiltrates, anemia, neutropenia | (13-17) |
| Mutation | |||||
| Afatinib | Irreversibly binds to the intracellular tyrosine kinase domain, subsequently inhibits EGFR, HER2 and HER4 receptors | Mutation | 15% (partial response rate) | Pulmonary toxicity, acute renal failure, hepatotoxicity, exfoliative dermatitis, hypokalemia | (19-21) |
| Dacomitinib | Irreversible binding at the ATP domain of the EGFR family kinase domains | Mutation | 12% (partial response rate) | Interstitial lung disease, diarrhea, rash, hypokalemia | (22) |
| Amplified | |||||
| TAK788 | EGFR antagonists; ERBB 2 receptor antagonists | Exon 20 mutation | 20–56% (partial response rate) | Diarrhea, nausea, rash, vomiting, decreased appetite | (28,29) |
| Poziotinib | Inhibitor of EGFR and HER2 exon 20 insertion mutants | Exon 20 mutation | 50% (partial response rate) | Rash, diarrhea, paronychia | (30,31) |
T-DM1, ado-trastuzumab emtansine; IHC, immunohistochemistry; HER2, human epidermal growth factor 2; AEs, adverse event; mAb, monoclonal antibody; CHF, congestive heart failure; EGFR, epidermal growth factor receptor.
Conclusions
Our patient showed an initial response to poziotinib reflecting the potential actionability of ErbB2 mutations in non-small cell lung cancer, however further data is necessary to assess its effectiveness in patients with the YVMA exon 20 insertion. Our case highlights the importance of developing agents that precisely target the various ErbB2 mutations, in particular the common YVMA exon 20 mutation, as these molecular alterations do not respond similarly to the various ErbB2-targeted agents.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Precision Cancer Medicine for the series “Precision Oncology Tumor Board”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pcm.2019.09.05). The Series “Precision Oncology Tumor Board” was commissioned by the editorial office without any funding or sponsorship. BH serves as the unpaid Guest Editor of the series and an unpaid editorial board member of Precision Cancer Medicine from Apr 2019 to Mar 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yarden Y. Biology of HER2 and its importance in breast cancer. Oncology 2001;61:1-13. [Crossref] [PubMed]
- Herter-Sprie GS, Greulich H, Wong KK. Activating Mutations in ERBB2 and Their Impact on Diagnostics and Treatment. Front Oncol 2013;3:86. [Crossref] [PubMed]
- Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177-82. [Crossref] [PubMed]
- Arcila ME, Chaft JE, Nafa K, et al. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin Cancer Res 2012;18:4910-8. [Crossref] [PubMed]
- Gazdar AF, Shigematsu H, Herz J, et al. Mutations and addiction to EGFR: the Achilles 'heal' of lung cancers? Trends Mol Med 2004;10:481-6. [Crossref] [PubMed]
- Greulich H, Kaplan B, Mertins P, et al. Functional analysis of receptor tyrosine kinase mutations in lung cancer identifies oncogenic extracellular domain mutations of ERBB2. Proc Natl Acad Sci U S A 2012;109:14476-81. [Crossref] [PubMed]
- Sun T, Aceto N, Meerbrey KL, et al. Activation of multiple proto-oncogenic tyrosine kinases in breast cancer via loss of the PTPN12 phosphatase. Cell 2011;144:703-18. [Crossref] [PubMed]
- Krishnamurti U, Silverman JF. HER2 in breast cancer: a review and update. Adv Anat Pathol 2014;21:100-7. [Crossref] [PubMed]
- Stephens P, Hunter C, Bignell G, et al. Lung cancer: intragenic ERBB2 kinase mutations in tumours. Nature 2004;431:525-6. [Crossref] [PubMed]
- Mar N, Vredenburgh JJ, Wasser JS. Targeting HER2 in the treatment of non-small cell lung cancer. Lung Cancer 2015;87:220-5. [Crossref] [PubMed]
- Yoshizawa A, Sumiyoshi S, Sonobe M, et al. HER2 status in lung adenocarcinoma: a comparison of immunohistochemistry, fluorescence in situ hybridization (FISH), dual-ISH, and gene mutations. Lung Cancer 2014;85:373-8. [Crossref] [PubMed]
- Pillai RN, Behera M, Berry LD, et al. HER2 mutations in lung adenocarcinomas: A report from the Lung Cancer Mutation Consortium. Cancer 2017;123:4099-105. [Crossref] [PubMed]
- Clamon G, Herndon J, Kern J, et al. Lack of trastuzumab activity in nonsmall cell lung carcinoma with overexpression of erb-B2: 39810: a phase II trial of Cancer and Leukemia Group B. Cancer 2005;103:1670-5. [Crossref] [PubMed]
- Gatzemeier U. Pemetrexed in malignant pleural mesothelioma. Oncology (Williston Park) 2004;18:26-31. [PubMed]
- Lara PN Jr, Chee KG, Longmate J, et al. Trastuzumab plus docetaxel in HER-2/neu-positive prostate carcinoma: final results from the California Cancer Consortium Screening and Phase II Trial. Cancer 2004;100:2125-31. [Crossref] [PubMed]
- Mazieres J, Peters S, Lepage B, et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol 2013;31:1997-2003. [Crossref] [PubMed]
- Mazieres J, Barlesi F, Filleron T, et al. Lung cancer patients with HER2 mutations treated with chemotherapy and HER2-targeted drugs: results from the European EUHER2 cohort. Ann Oncol 2016;27:281-6. [Crossref] [PubMed]
- Li BT, Shen R, Buonocore D, et al. Ado-Trastuzumab Emtansine for Patients With HER2-Mutant Lung Cancers: Results From a Phase II Basket Trial. J Clin Oncol 2018;36:2532-7. [Crossref] [PubMed]
- De Greve J, Teugels E, Geers C, et al. Clinical activity of afatinib (BIBW 2992) in patients with lung adenocarcinoma with mutations in the kinase domain of HER2/neu. Lung Cancer 2012;76:123-7. [Crossref] [PubMed]
- Li BT, Lee A, O'Toole S, et al. HER2 insertion YVMA mutant lung cancer: Long natural history and response to afatinib. Lung Cancer 2015;90:617-9. [Crossref] [PubMed]
- Lai WV, Lebas L, Barnes TA, et al. Afatinib in patients with metastatic or recurrent HER2-mutant lung cancers: a retrospective international multicentre study. Eur J Cancer 2019;109:28-35. [Crossref] [PubMed]
- Kris MG, Camidge DR, Giaccone G, et al. Targeting HER2 aberrations as actionable drivers in lung cancers: phase II trial of the pan-HER tyrosine kinase inhibitor dacomitinib in patients with HER2-mutant or amplified tumors. Ann Oncol 2015;26:1421-7. [Crossref] [PubMed]
- Gandhi L, Bahleda R, Tolaney SM, et al. Phase I study of neratinib in combination with temsirolimus in patients with human epidermal growth factor receptor 2-dependent and other solid tumors. J Clin Oncol 2014;32:68-75. [Crossref] [PubMed]
- Liu S, Li S, Hai J, et al. Targeting HER2 Aberrations in Non-Small Cell Lung Cancer with Osimertinib. Clin Cancer Res 2018;24:2594-604. [Crossref] [PubMed]
- Salem A, Asselin MC, Reymen B, et al. Targeting Hypoxia to Improve Non-Small Cell Lung Cancer Outcome. J Natl Cancer Inst 2018; [Crossref] [PubMed]
- Estrada-Bernal A, Doak AE, Le AT, et al. Abstract A157: Antitumor activity of tarloxotinib, a hypoxia-activated EGFR TKI, in patient-derived lung cancer cell lines harboring EGFR exon 20 insertions. In: Proceedings of the AACR-NCI-EORTC International Conference: Molecular Targets and Cancer Therapeutics, 2017.
- Doebele RC, Riely GJ, Spira AI, et al. First report of safety, PK, and preliminary antitumor activity of the oral EGFR/HER2 exon 20 inhibitor TAK-788 (AP32788) in non–small cell lung cancer (NSCLC). J Clin Oncol 2018;36:abstr 9015.
- Janne PA, Neal JW, Camidge DR, et al. Antitumor activity of TAK-788 in NSCLC with EGFR exon 20 insertions. J Clin Oncol 2019;37:abstr 9007.
- ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Identifier NCT02716116.
- Robichaux JP, Elamin YY, Tan Z, et al. Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer. Nat Med 2018;24:638-46. [Crossref] [PubMed]
- Heymach J, Negrao M, Robichaux J, et al. OA02.06 A Phase II Trial of Poziotinib in EGFR and HER2 exon 20 Mutant Non-Small Cell Lung Cancer (NSCLC). J Thorac Oncol 2018;13:S323-4. [Crossref]
Cite this article as: Ge X, Tariq S, Cheng H, Halmos B. Targeting ErbB2 aberrations in non-small cell lung cancer. Precis Cancer Med 2019;2:27.

