
Low-dose lung cancer screening: nodule measurement and management
Introduction
Lung cancer has the highest mortality rate of all cancers. It is estimated that annually 1.6 million deaths are due to lung cancer worldwide, making it the leading cause of cancer-related death (1-3). The time of diagnosis directly translates to survival, with 5-year survival rates ranging between 12–90% depending on the lung cancer stage (4). An important factor is that lung cancer often remains without symptoms until far progressed and most clinical patients are beyond stage I (4). Several studies, including randomized controlled trials, have examined or are examining the possibility of lung cancer screening with low-dose computed tomography (LDCT) to reduce mortality (5,6). After the American National Lung Screening Trial (NLST) showed a 20% reduced lung cancer mortality when comparing LDCT to conventional chest radiography (7), most US guidelines now recommend LDCT lung cancer screening for high-risk individuals (8-10). European stakeholders, however, are awaiting the final results of the Dutch-Belgian lung cancer screening (NELSON) trial (9,11).
In lung cancer screening trials, around 22–51% of participants have non-calcified pulmonary nodules and their detection and management defines the success of any lung cancer screening program (12-21). This review focusses on current clinical challenges in nodule measurement and risk-stratification of baseline and incident nodules.
Measurement of nodule size and nodule growth
Lung nodule management is based on nodule size and growth rate (22-24). Hence, accurate and reproducible nodule measurement is directly related to the success of a lung cancer screening program. Traditionally, nodule size has been determined using calipers, measuring average diameter rounded to the nearest whole number for non-spherical nodules and a single diameter for spherical nodules (22,24). However, since pulmonary nodules seldom are perfectly geometrically shaped, errors in nodule sizing may result (25,26). Unfortunately, this especially concerns nodules with a non-smooth margin, which have a higher lung cancer probability than smooth nodules (25,27-30). A more recent approach, applied by several European lung cancer screening trials, is the semi-automated measurement of nodule volume using software (5,17,31). This enables an estimation of nodule size after 3-dimensional reconstruction of thin slices (maximum 1 mm to create isometric voxels) and was also applied in guidelines from the British Thoracic Society and Fleischner society (22,23,32).
Importantly, most nodules detected during lung cancer screening are small. The NELSON trial showed, that around 60% of new solid nodules detected were smaller than 50 mm3 (roughly 4.5 mm) (31,33). The ELCAP and Mayo trial reported similar numbers between 40–55% for new nodules smaller than 5 and 4 mm respectively (34,35). At these tiny nodule sizes, growth detection based on two-dimensional diameter evaluation is very unreliable (32), especially in comparison to volumetry. Additionally, even a computer simulated mean diameter provided inferior performance for nodule risk-stratification when compared to semi-automated volume measurement (31).
During follow-up screenings nodule growth can be estimated based on the detected change in size. The nodule management algorithm currently employed in the USA defines growth as an increase in mean diameter of >1.5 mm, while assuming equal growth in all directions (24,32). Nevertheless, it was found that already for intermediately sized nodules (50–500 mm3, roughly 4.5–10 mm), intra-nodular diameter, defined as the difference between maximum and minimum diameter in any direction, variation varied by 2.8 mm, which is above the 1.5 mm cutoff (26). Even when measuring the diameter semi-automatically, thereby limiting inaccuracy of human readers, 85% of nodules have an intra-nodular diameter variation of at least 2 mm, which transfers them between different lung cancer probability scores and therefore different management categories ranging from regular screening to short-term imaging (26,32,36).
Rather than using a fixed cutoff for size increase, the growth rate or volume doubling time (VDT) can be estimated based on the screening interval and change in size. Considering that lung cancers typically grow according to exponential growth patterns (37,38), VDT instead of a fixed increase is far more accurate and therefore appropriate for nodule management. A retrospective analysis of the NELSON study showed that, compared to optimized diameter-based protocols, an optimized protocol based on semi-automated nodule volume yielded the highest specificity and positive predictive value with similar negative predictive value (32,39). Again, in this study the radiologist was aided by three-dimensional software to assess the nodule diameter and manual diameter measurements probably would have performed even worse.
While there remain uncertainties and limitations of semi-automated volume measurements, such as the occasional need to manually adapt the segmented volume, this also applies to diameter measurement. For instance, nodule attachment and nodule margin can cause variability in manual diameter measurements between different radiologists which potentially leads to an increase in false positives in terms of growth determination (36). Currently, evidence shows that management of (solid) nodules detected in lung cancer screening has to be based on semi-automated nodule volume and VDT, while nodule diameter measurements should only be used where volumetry is not technically possible (11).
Management of pulmonary nodules in lung cancer screening
Pulmonary nodules are regularly found in CT lung cancer screening. European and American trials without a detection limit or with a low detection limit (3 mm or 15 mm3) found a non-calcified pulmonary nodule prevalence in between 41–51% of participants at baseline with up to 56% of these nodules being small pulmonary nodules below 50 mm3 or 4.5 mm (12-15,33). Since most of these nodules are benign, the accurate identification of high-risk nodules that require immediate referral for diagnostic work-up as well as the identification of low-risk nodules is key to any screening program. Underestimating the risk may cause delayed lung cancer diagnosis, thereby effectively increasing the mortality of participants (4,22,40). Conversely, a risk overestimation may cause unnecessary and potentially harmful procedures (2,10,41).
Nodules detected at baseline potentially have been present for years, whereas nodules newly developed after baseline are possibly fast-growing. Considering that the timeframe in which a new nodule developed is known, the size at detection might be used to estimate its growth speed (31). Until recently, there was only very limited evidence concerning the risk-stratification of new nodules detected after baseline (8,23,31). Consequently, new nodule management was mostly based on expert-opinion or data derived from baseline nodules (8,23,24,42,43).
In case of baseline nodules, it has been established that nodule size is the most important predictor for lung cancer. After it was shown that the 2-year lung cancer probability for participants without baseline nodules did not differ significantly from participants with a baseline nodule <100 mm3 (lung cancer probability 0.6%) (39), this has been advocated as low risk-threshold for baseline nodules (11). Conversely, lung cancer probability rose sharply in participants with a baseline nodule >300 mm3 (lung cancer probability >16.9%) leading to a recommendation for direct referral (11,39). Similar recommendations have been made based on diameter lung cancer screening studies (22,24,44).
Management of new incident nodules in lung cancer screening
Recent evidence from the NELSON trial shows that new nodules should be managed differently from baseline nodules (11,31,45). In annual screening, 5% of the NELSON participants developed a new non-calcified solid nodule, while 11% of the NELSON participants developed a new non-calcified solid nodule within the first two incidence screening rounds (3 years after baseline) (31). The annual new solid nodule occurrence was similar to numbers from the ELCAP and IELCAP studies (3% and 5% respectively) as well as the PLuSS trial (7%) (12,19,34). In response to the NELSON trial’s results, an analysis of the NLST reported an annual incidence of new nodules in around 3% of participants [note: only nodules ≥4 mm were registered as compared to 15 mm3 (2.9 mm) in the NELSON trial] (45). Nevertheless, these numbers are limited in their direct comparability, as new nodules were defined differently within trials and rates have not been reported explicitly (8,33). Compared to new solid nodules, non-solid new nodules are less common. New subsolid nodules were found in <1% of NELSON participants with at least one screening after baseline (46). This is comparable to findings of the I-ELCAP trial, where <1% of participants presented with new part-solid or new non-solid nodules respectively (47,48).
The lung cancer probability of new solid nodules is higher than in baseline nodules. It was found that 6% of participants with a new non-calcified solid nodule developed lung cancer in such a nodule, with 4% of the new solid nodules proving to be lung cancer (31,33). In a previous analysis of the NELSON trial, 1% of participants were detected with lung cancer during the baseline screening round (14), and for the first three rounds it was reported that approximately 3% of participants were detected with lung cancer (including new nodule cancer) (49). The ELCAP trial reported that 10% of participants with new non-calcified pulmonary incident nodules on LDCT had lung cancer in a new nodule, and the IELCAP reported this was the case for 5% of its participants (19,34). In response to the NELSON trial’s analysis, data of the NLST confirmed the results, with 6% of the new solid nodules being lung cancer as compared to only 3% of the baseline solid nodules (45,50).
The lung cancer probability of new subsolid nodules is similar to those of baseline nodules. It was found that 6% of NELSON participants with a new subsolid nodule had a lung cancer diagnosed in such a nodule, with 5% of the new subsolid nodules being lung cancer (46,51). Until now the I-ELCAP is the only other trial that reported new subsolid nodule lung cancer frequencies. Overall, 4% of the new subsolid nodules detected in the I-ELCAP trial were lung cancer (47,48). All new subsolid nodule lung cancers detected in the NELSON trial were stage I or adenocarcinoma in situ (46,51). This is comparable to results of the I-ELCAP trial where all new subsolid lung cancer cases were stage I (47,48). Additionally, previous prospective Japanese studies reported that all pathologically confirmed tumors in subsolid nodules were stage I and ≤1% of subsolid nodules were invasive adenocarcinomas (46,51-53). Furthermore, an analysis of lung cancer manifesting as nonsolid nodule (baseline and incident clustered together) in the NLST confirms this (54).
There only is little evidence from other lung cancer screening trials concerning the stratification of new solid nodules. However, new nodule size at initial detection provides moderate to high discriminative ability for lung cancer (31). Comparing the second NELSON screening round (1 year screening interval) and the third NELSON screening round (2-year screening interval), the discriminative power increased with a longer screening interval (31). This suggests that new nodules need time to grow in order to be evaluated based on size only, making growth speed measures such as the VDT imperative for follow-up assessment.
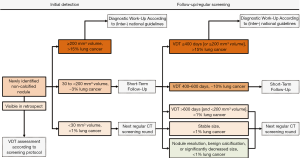
The new solid nodule lung cancer probability is high at a small size, especially in comparison with nodules detected at baseline (31). Prior to the presented studies, data of the NELSON trial suggested that baseline nodules smaller than 100 mm3 had a 2-year lung cancer probability of about 0.6%, were not predictive of lung cancer, and did not necessitate additional follow-up scans. However, this criterion does not apply in the case of new solid nodules. In case of new nodules, 3% of participants whose largest new solid nodule was smaller than 100 mm3 were eventually diagnosed with lung cancer, with 2% of new solid nodules smaller than 100 mm3 found to be lung cancer (31). These findings were later confirmed by an analysis of the NLST which, at smaller size, reported a significantly higher lung cancer risk in new solid nodules as compared to baseline nodules (45). The optimized volume cutoffs for new solid nodules were <27 mm3 (<1% lung cancer probability, low risk), 27–206 mm3 (3% lung cancer probability, intermediate risk), and ≥206 mm3 (17% lung cancer probability, high risk), providing 95% sensitivity (31). It has been proposed by a European expert group to adapt these cutoffs for clinical implementation to 30 and 200 mm3 respectively (11). The optimized computer simulated mean diameter cutoffs were <3.7 mm (<1% lung cancer probability, low risk), 3.7–8.2 mm (3% lung cancer probability, intermediate risk), and ≥8.2 mm (14% lung cancer probability, high risk), providing 95% sensitivity (31). These threshold probabilities are implemented in concordance with the lung cancer probabilities for the respective American College of Radiologists Lung-RADS categories (24). The results of the NELSON trial were subsequently confirmed by data of the NLST (45). It was found that especially at very small size, the lung cancer risk between new solid nodules and baseline nodules differed markedly: 4–6 mm mean diameter (2.3% vs. 0.4%, 6 times higher) and 6–8 mm mean diameter (5.4% vs. 1.3%, 4 times higher) (45). Even more remarkably, while the NLST did not include nodules with a longest diameter <4 mm (around 34 mm3) and therefore cannot represent these nodules accurately, the researchers reported a significantly higher lung cancer risk in nodules with a mean diameter <4 mm (1.1% vs. 0.1%) (45). In light of these results, the currently advocated, new solid nodule size diameter cutoff in LungRADs of 4 mm (24), should be reviewed to assess whether it appropriately represents the actual lung cancer risk of new solid nodules. At follow-up after new solid nodule detection, volume provided high and VDT provided very high discrimination for lung cancer (55). The performance was higher than at initial detection (31,55), underlining that the discrimination of new nodule lung cancers increases with longer screening time interval. The addition of the previously found high-risk volume cutoff of 200 mm3 further improved discrimination by VDT alone (55). Of new solid nodules <200 mm3 at initial detection and ≥200 mm3 at first subsequent screening LDCT, 38% were lung cancer and the addition of a volume limit could improve risk stratification also after initial detection (55). Considering that lung cancer growth was shown to not always be exponential or linear (37,38), addition of a volume limit compelling referral to a pulmonologist might prevent slow growing lung cancers from evading timely referral. The optimized VDT cutoff was 590 days and combined with the ≥200 mm3 high-risk cutoff, thereby classifying nodules positive when at least one criterion was fulfilled, provided 100% sensitivity and 84% specificity for discriminating lung cancer (55). The observed statistically optimal VDT cutoff of ≤590 days is analogous to currently employed cutoffs of ≤600 days (11,23).
Importantly, more than half (55%) of new nodules resolve until first follow-up after initial detection (55). In total, 7% of participants with non-resolving low- and intermediate risk new solid nodules (0–3% lung cancer probability based on risk-stratification at initial detection) had lung cancer in such a nodule (55). Moreover, with longer screening interval the number of new nodules does not increase proportionally while the proportion of lung cancers further increases (31). This phenomenon could be explained by the nature of non-resolving new nodules: The longer a screening interval, the higher the proportion of non-resolving new nodules and consequently the higher the percentage of lung cancers (55). This is important when assessing new nodules found after different screening interval lengths or during short-term follow-up. A previous study of the NELSON trial examined the disappearance of intraparenchymal solid baseline nodules sized 50–500 mm3 and reported that 90% of the nodules persisted, with 3% of non-resolving nodules being diagnosed as lung cancer eventually (56). The fact that compared to baseline nodules more new nodules resolve can be explained by the difference of the two nodule groups. Baseline nodules may have been present for years and are therefore more likely to be stable, while new nodules develop within a short, known time-frame and are more likely dynamic. Thus, the mere persistence of a new nodule might be considered as a risk factor for lung cancer.
Therefore, while new subsolid nodules can be managed equivalent to baseline subsolid nodules, new solid nodules require a more aggressive approach as compared to baseline nodules. Figure 1 summarizes the presented evidence for the management of new solid nodules.
Extending lung cancer screening
Until now, cost-effectiveness in LDCT lung cancer screening programs, was driven primarily by non-lung cancer outcomes, including improvement in the quality of life and smoking cessation (57,58). It has been established that lung cancer, chronic obstructive pulmonary disease, and cardiovascular disease share risk-factors and are highly prevalent in the population (57). Considering the possibilities of chest CT screening including emphysema screening, cardiac calcium scoring and lung cancer screening, integrating all three acquisition protocols into one scan could be worthwhile (57). While this hypothesis requires further prospective studies, it offers an opportunity to maximize health benefits and cost-effectiveness of screening.
Conclusions
Lung cancer screening programs are ongoing and challenges remain. Semi-automated volume measurement can be superior to diameter measurement, but is currently limited by its availability. New nodules that develop after baseline require lower size cutoff values and therefore limit an accurate stratification by diameter protocols. The clinical implementation of these findings is ongoing.
Acknowledgments
This manuscript contains data from JE Walter’s PhD thesis.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor Nir Peled for the series dedicated to the Congress on Clinical Controversies in Lung Cancer (CCLC 2018) published in Precision Cancer Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pcm.2019.07.03). The series dedicated to the Congress on Clinical Controversies in Lung Cancer (CCLC 2018) was commissioned by the editorial office without any funding or sponsorship. The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- van der Aalst CM, ten Haaf K, de Koning HJ. Lung cancer screening: latest developments and unanswered questions. Lancet Respir Med 2016;4:749-61. [Crossref] [PubMed]
- Stewart BW, Wild C, International Agency for Research on Cancer, World Health Organization. World Cancer Report 2014.
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Field JK, Van Klaveren R, Pedersen JH, et al. European randomized lung cancer screening trials: Post NLST. J Surg Oncol 2013;108:280-6. [Crossref] [PubMed]
- Midthun DE, Jett JR. Screening for lung cancer: The US studies. J Surg Oncol 2013;108:275-9. [Crossref] [PubMed]
- Aberle DR, Adams AM, Berg CD, et al. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Bach PB, Mirkin JN, Oliver TK, et al. Benefits and Harms of CT Screening for Lung Cancer. JAMA 2012;307:2418. [Crossref] [PubMed]
- Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2017: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin 2017;67:100-21. [Crossref] [PubMed]
- de Koning HJ, Meza R, Plevritis SK, et al. Benefits and harms of computed tomography lung cancer screening strategies: A comparative modeling study for the U.S. Preventive services task force. Ann Intern Med 2014;160:311-20. [Crossref] [PubMed]
- Oudkerk M, Devaraj A, Vliegenthart R, et al. European position statement on lung cancer screening. Lancet Oncol 2017;18:e754-66. [Crossref] [PubMed]
- Wilson DO, Weissfeld JL, Fuhrman CR, et al. The Pittsburgh lung screening study (PLuSS): Outcomes within 3 years of a first computed tomography scan. Am J Respir Crit Care Med 2008;178:956-61. [Crossref] [PubMed]
- Swensen SJ, Jett JR, Hartman TE, et al. CT screening for lung cancer: five-year prospective experience. Radiology 2005;235:259-65. [Crossref] [PubMed]
- van Klaveren RJ, Oudkerk M, Prokop M, et al. Management of Lung Nodules Detected by Volume CT Scanning. N Engl J Med 2009;361:2221-9. [Crossref] [PubMed]
- Field JK, Duffy SW, Baldwin DR, et al. UK Lung Cancer RCT Pilot Screening Trial: baseline findings from the screening arm provide evidence for the potential implementation of lung cancer screening. Thorax 2016;71:161-70. [Crossref] [PubMed]
- Henschke CI, McCauley DI, Yankelevitz DF, et al. Early Lung Cancer Action Project: Overall design and findings from baseline screening. Lancet 1999;354:99-105. [Crossref] [PubMed]
- Pedersen JH, Ashraf H, Dirksen A, et al. The Danish randomized lung cancer CT screening trial--overall design and results of the prevalence round. J Thorac Oncol 2009;4:608-14. [Crossref] [PubMed]
- Church TR, Black WC, Aberle DR, et al. Results of Initial Low-Dose Computed Tomographic Screening for Lung Cancer. N Engl J Med 2013;368:1980-91. [Crossref] [PubMed]
- Henschke CI, Yankelevitz DF, McCauley DI, et al. Survival of Patients with Stage I Lung Cancer Detected on CT Screening. N Engl J Med 2006;355:1763-71. [Crossref] [PubMed]
- Lopes Pegna A, Picozzi G, Mascalchi M, et al. Design, recruitment and baseline results of the ITALUNG trial for lung cancer screening with low-dose CT. Lung Cancer 2009;64:34-40. [Crossref] [PubMed]
- Becker N, Motsch E, Gross ML, et al. Randomized study on early detection of lung cancer with MSCT in Germany: Study design and results of the first screening round. J Cancer Res Clin Oncol 2012;138:1475-86. [Crossref] [PubMed]
- MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017;284:228-43. [Crossref] [PubMed]
- Callister MEJ, Baldwin DR, Akram AR, et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax 2015;70:ii1-54. [Crossref] [PubMed]
- American College of Radiology. LungRADSTM Version 1.0 Assessment Categories Release date: April 28, 2014. 2014.
- Han D, Heuvelmans MA, Vliegenthart R, et al. Influence of lung nodule margin on volume-and diameter-based reader variability in CT lung cancer screening. Br J Radiol 2018;91:20170405. [Crossref] [PubMed]
- Heuvelmans MA, Walter JE, Vliegenthart R, et al. Disagreement of diameter and volume measurements for pulmonary nodule size estimation in CT lung cancer screening. Thorax 2018;73:779-81. [Crossref] [PubMed]
- Walter JE, Heuvelmans MA, De Bock GH, et al. Characteristics of new solid nodules detected in incidence screening rounds of low-dose CT lung cancer screening: The NELSON study. Thorax 2018;73:741-7. [Crossref] [PubMed]
- McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med 2013;369:910-9. [Crossref] [PubMed]
- Xu DM, van der Zaag-Loonen HJ, Oudkerk M, et al. Smooth or attached solid indeterminate nodules detected at baseline CT screening in the NELSON study: cancer risk during 1 year of follow-up. Radiology 2009;250:264-72. [Crossref] [PubMed]
- Xu DM, van Klaveren RJ, de Bock GH, et al. Limited value of shape, margin and CT density in the discrimination between benign and malignant screen detected solid pulmonary nodules of the NELSON trial. Eur J Radiol 2008;68:347-52. [Crossref] [PubMed]
- Walter JE, Heuvelmans MA, de Jong PA, et al. Occurrence and lung cancer probability of new solid nodules at incidence screening with low-dose CT: Analysis of data from the randomised, controlled NELSON trial. Lancet Oncol 2016;17:907-16. [Crossref] [PubMed]
- Heuvelmans MA, Oudkerk M. Pulmonary nodules measurements in CT lung cancer screening. J Thorac Dis 2018;10:S2100-2. [Crossref] [PubMed]
- Walter JE, Heuvelmans MA, Oudkerk M. Small pulmonary nodules in baseline and incidence screening rounds of low-dose CT lung cancer screening. Transl lung cancer Res 2017;6:42-51. [Crossref] [PubMed]
- Henschke CI, Naidich DP, Yankelevitz DF, et al. Early Lung Cancer Action Project: Initial findings on repeat screening. Cancer 2001;92:153-9. [Crossref] [PubMed]
- Swensen SJ, Jett JR, Sloan JA, et al. Screening for lung cancer with low-dose spiral computed tomography. Am J Respir Crit Care Med 2002;165:508-13. [Crossref] [PubMed]
- Han D, Heuvelmans MA, Vliegenthart R, et al. Influence of lung nodule margin on volume- and diameter-based reader variability in CT lung cancer screening. Br J Radiol 2018;91:20170405. [Crossref] [PubMed]
- Lindell RM, Hartman TE, Swensen SJ, et al. 5-Year lung cancer screening experience: Growth curves of 18 lung cancers compared to histologic type, CT attenuation, stage, survival, and size. Chest 2009;136:1586-95. [Crossref] [PubMed]
- Heuvelmans MA, Vliegenthart R, de Koning HJ, et al. Quantification of growth patterns of screen-detected lung cancers: The NELSON study. Lung Cancer 2017;108:48-54. [Crossref] [PubMed]
- Horeweg N, van Rosmalen J, Heuvelmans MA, et al. Lung cancer probability in patients with CT-detected pulmonary nodules: A prespecified analysis of data from the NELSON trial of low-dose CT screening. Lancet Oncol 2014;15:1332-41. [Crossref] [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the TNM Stage Groupings in the Forthcoming (Seventh) Edition of the TNM Classification of Malignant Tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Patz EF, Pinsky P, Gatsonis C, et al. Overdiagnosis in Low-Dose Computed Tomography Screening for Lung Cancer. JAMA Intern Med 2014;174:269. [Crossref] [PubMed]
- Aberle DR, Berg CD, Black WC, et al. The National Lung Screening Trial: Overview and Study Design. Radiology 2011;258:243-53. [Crossref] [PubMed]
- Xu DM, Gietema H, de Koning H, et al. Nodule management protocol of the NELSON randomised lung cancer screening trial. Lung Cancer 2006;54:177-84. [Crossref] [PubMed]
- Henschke CI, Yip R, Yankelevitz DF, et al. Definition of a Positive Test Result in Computed Tomography Screening for Lung Cancer. Ann Intern Med 2013;158:246. [Crossref] [PubMed]
- Pinsky PF, Gierada DS, Hrudaya Nath P, et al. Lung cancer risk associated with new solid nodules in the national lung screening trial. AJR Am J Roentgenol 2017;209:1009-14. [Crossref] [PubMed]
- Walter JE, Heuvelmans MA, Yousaf-Khan U, et al. New Subsolid Pulmonary Nodules in Lung Cancer Screening: The NELSON Trial. J Thorac Oncol 2018;13:1410-4. [Crossref] [PubMed]
- Henschke CI, Yankelevitz DF, Mirtcheva R, et al. CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. AJR Am J Roentgenol 2002;178:1053-7. [Crossref] [PubMed]
- Henschke CI, Yip R, Smith JP, et al. CT Screening for Lung Cancer: Part-Solid Nodules in Baseline and Annual Repeat Rounds. AJR Am J Roentgenol 2016;207:1176-84. [Crossref] [PubMed]
- Horeweg N, Van Der Aalst CM, Thunnissen E, et al. Characteristics of lung cancers detected by computer tomography screening in the randomized NELSON trial. Am J Respir Crit Care Med 2013;187:848-54. [Crossref] [PubMed]
- Aberle DR, DeMello S, Berg CD, et al. Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med 2013;369:920-31. [Crossref] [PubMed]
- Heuvelmans MA, Walter JE, Oudkerk M. Management of baseline and new sub-solid nodules in CT lung cancer screening. Expert Rev Respir Med 2018;12:1-3. [Crossref] [PubMed]
- Kakinuma R, Muramatsu Y, Kusumoto M, et al. Solitary Pure Ground-Glass Nodules 5 mm or Smaller: Frequency of Growth. Radiology 2015;276:873-82. [Crossref] [PubMed]
- Kakinuma R, Noguchi M, Ashizawa K, et al. Natural history of pulmonary subsolid nodules: A prospective multicenter study. J Thorac Oncol 2016;11:1012-28. [Crossref] [PubMed]
- Yip R, Yankelevitz DF, Hu M, et al. Lung Cancer Deaths in the National Lung Screening Trial Attributed to Nonsolid Nodules. Radiology 2016;281:589-96. [Crossref] [PubMed]
- Walter JE, Heuvelmans MA, Ten Haaf K, et al. Persisting new nodules in incidence rounds of the NELSON CT lung cancer screening study. Thorax 2019;74:247-53. [Crossref] [PubMed]
- Zhao YR, Heuvelmans MA, Dorrius MD, et al. Features of Resolving and Nonresolving Indeterminate Pulmonary Nodules at Follow-up CT: The NELSON Study. Radiology 2014;270:872-9. [Crossref] [PubMed]
- Heuvelmans MA, Vonder M, Rook M, et al. Screening for Early Lung Cancer, Chronic Obstructive Pulmonary Disease, and Cardiovascular Disease (the Big-3) Using Low-dose Chest Computed Tomography. J Thorac Imaging 2019;34:160-9. [Crossref] [PubMed]
- Cressman S, Peacock SJ, Tammemägi MC, et al. The Cost-Effectiveness of High-Risk Lung Cancer Screening and Drivers of Program Efficiency. J Thorac Oncol 2017;12:1210-22. [Crossref] [PubMed]
Cite this article as: Walter JE, Heuvelmans MA, Dorrius M, Oudkerk M. Low-dose lung cancer screening: nodule measurement and management. Precis Cancer Med 2019;2:24.

