Planning and marking small nodules for surgery
Introduction
Early lung cancer diagnosis results of paramount importance nowadays. This main topic involves having to deal with very small lesions, but, on the other hand, thoracic surgery tends to be developed through minimal invasive techniques.
Localization of small pulmonary nodules (less than 10 mm in diameter) through minimally invasive approaches is challenging because some of those identified on computed tomography (CT) are not visible or palpable during surgery (1). For this reason, several preoperative localization methods have been developed to reduce thoracotomy conversion rate associated with the difficulty of localising small nodules through minimally invasive approaches.
Two different types of perioperative localising methods have been described: (I) percutaneous techniques using hook-wires, contrast media and radiotracers inserted into the nodule or in a perinodular location; (II) bronchoscopic techniques mainly by endobronchial electromagnetic navigation.
We describe in this issue techniques and pros and cons of each method, emphasizing results and applicability.
We also point out the relevance in modern surgery of hybrid operating rooms (HOR): These are rooms that lodge interventional radiology technology and surgical equipment allowing for a combined use of all that technology on one patient in the same place and at the same time.
Techniques to identify pulmonary nodules
Transthoracic procedures for localizing small pulmonary nodules
These methods consist of techniques wherein percutaneous CT-guided localizers, including hook-wires, contrast media or radiotracers are inserted into the nodule or in a perinodular location. In these methods, the nodule is initially localized by CT in the radiology department, followed by surgical resection in an operating room (OR).
Hook-wire localization
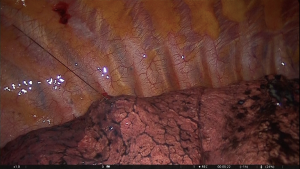
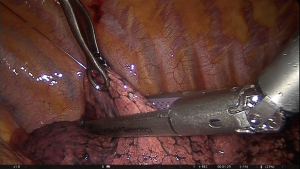
The technique consists of preoperative placement of a localization wire into the nodule while it is under CT scan control. It is the oldest and probably the most common method of nodule localization (2). The system includes a folded hook-wire enclosed within the 20G guidance needle. Optimal puncture site, angle and route are determined from previous CT-scans. Following local anaesthesia of the puncture site, the tip of the needle is positioned as close as possible to the nodule and the hook-wire spreads its anchoring hooks by withdrawing the guidance needle (3). The wire is easily visible during the thoracoscopic procedure without intraoperative fluoroscopy and radiation exposure (Figures 1,2). The reported successful rate is around 93.6–97.6% (4-9). The major disadvantage of this method is the hook-wire dislodgement from a perinodular location. The reported dislodgement rate is 2.4–6.9% (4-9). Moreover, there are some anatomical locations (apical, diaphragmatic and near the great vessels) that would be a limitation for the procedure. Among potential complications, minor pneumothorax (7.5–40%), lung parenchyma haemorrhage (13.9–36%) and subcutaneous emphysema (5%) have been described (3-9). Pain between puncture time and surgery is one of the main disadvantages of this method.
Microcoil and fiducial marker placement
The technique consists of preoperative placement of platinum microcoils into the nodule under CT-guidance. No wire is left protruding extracorporeally after CT-guided localization, so that it may decrease the discomfort of patients during the waiting time before the surgical resection. The procedure is similar to that for hook-wire localization. Using local anaesthesia in the puncture site, the microcoil is passed through a coaxial needle and under CT-guidance the distal end of the microcoil is placed deep to the nodule and the superficial end coiled on the visceral pleural surface. The superficial coiled end is easily visualized during the video-assisted thoracic surgery (VATS) procedure guiding to the deep coil end attached to the nodule (10). However, fiducial marker localization usually requires fluoroscopic guidance. The success rate is 93–98.4% (11-15). Microcoil and fiducial marker migration may occur in 3-10% of patients (11-15). Main complications include air embolism, marker embolization, focal intrapulmonary haemorrhage, pneumothorax and haemothorax.
Lipiodol injection
The technique consists of the injection of water-soluble contrast medium such as barium and lipiodol (16,17) within or around the lung nodule. The contrast media can be injected by CT-guided needle injection or by CT-guided bronchoscopy injection. The main advantages of lipiodol is that it diffuses to a very small area in the lung parenchyma and is retained in the lung parenchyma for a long time (up to 3 months) so that the patient does not need to hurry to the OR immediately. The marked nodule can be intraoperatively detected by fluoroscopy and the reported success rate is 100% (16,17). In addition, unlike other techniques, lipiodol marking permits resection of lesions while verifying the stapled margin in real time (18). Postprocedural complications such as pneumothorax and haemorrhage can occur, however most such events can be resolved with conservative management.
Other techniques
Methylene blue dye localization
The success rate of localization of pulmonary nodules by methylene blue is high and the localization procedure time is short. The major disadvantage of this procedure is that the methylene blue dye may rapidly diffuse into the surrounding lung parenchyma.
Radiotracer-guided localization
Radiotracer-guided localization uses gamma-emitting radioisotopes (technetium 99) attached to albumin molecules which are injected percutaneously into the nodule under CT-guidance (19-21). Gamma-ray emissions can be detected intraoperatively by a probe converting them into digital counts as well as audio signals. The area with the strongest signal can be identified as the lesion site. The disadvantage is that this technique is highly facility-dependent due to the radiotracer, gamma probe, and radiation protection equipment.
Among these techniques, the three most widely used localization methods are CT-guided hook-wire localization, microcoil localization and lipiodol localization. Recently a systematic review and meta-analysis has evaluated the pooled success and complications rates of these three methods (22). The overall mean success rate of hook-wire localization was 0.96 (95% CI, 0.95–0.97). For microcoil localization and lipiodol localization, the mean success rates were 0.97 (95% CI, 0.94–0.98) and 0.99 (95% CI, 0.97–1.00), respectively. Regarding the complications rates, the mean pneumothorax rates associated with hook-wire, microcoil, and lipiodol localization were 0.35 (95% CI, 0.28–0.43), 0.16 (95% CI, 0.07–0.34), and 0.31 (95% CI, 0.20–0.46), respectively. The mean haemorrhage rates associated with hook-wire, microcoil, and lipiodol localization were 0.16 (95% CI, 0.11–0.23), 0.06 (95% CI, 0.03–0.11), and 0.12 (95% CI, 0.05–0.23), respectively.
Electromagnetic navigation bronchoscopy (ENB)
We have previously explained transthoracic procedures to mark small pulmonary nodules and its several disadvantages such as pain, inaccuracy...
ENB could be deemed as an ideal method for nodule localization because it has a high grade of accuracy and can be performed in the same operative setting as minimally invasive surgery assuming a minimal risk for the patient, and under general anaesthesia.
We should consider ENB for those nodules smaller than 1 cm independently of its location or those smaller than 2 cm which are further than 1 cm from pleural surface.
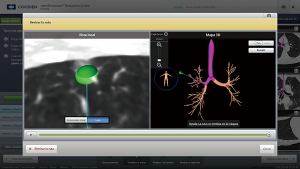
While planning ENB, we must assume some premises like having appropriate CT scan matching ENB software. ENB superDimension software converts two-dimensional preoperative CT images into a three-dimensional (3D) virtual bronchial map (Figure 3). We use that map to identify the quickest path to the nodule. The procedure is performed under general anaesthesia and a single-lumen endotracheal tube or a laryngeal mask is placed. After that, conventional bronchoscopy to the level of segmental bronchi must be accomplished. Then, ENB is performed, and navigation to the previously identified lesions in the planning phase is completed. During the procedure, a dirigible bronchoscopic device with a sensor probe communicates with an electromagnetic board generating a field in which probe’s position can be sensed and we can follow it in our virtual bronchial map. The locatable guide is removed and the extended working channel is used by standard flexible bronchoscopic needle to the target lesion (previously primed with methylene blue or any other contrast we are employing).
It would be an ideal condition to present a bronchus leading in the lesion. But when there is no bronchus leading to the nodule, we must consider the best available exit point from the bronchial tree. Even when it does not coincide exactly with the nodule, a reference is taken to resect the lesion. If it is available, a hybrid approach with CT assessment should be employed not only to assure the actual point of dye marking, but also to help guide depth of resection (23).
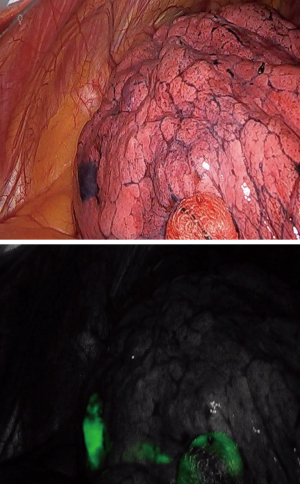
Dye marking could be performed with methylene blue, isocyanine green (ICG) or fiducials. Whether ICG is the preferred technique a specific ICG camera is mandatory (Figure 4).
Methylene blue has some advantages like its easy reproducibility, its availability and its inexpensive cost. Recommended injected volume varies in literature from 0.5 to 1 mL (24). In our centre, 1 mL is the volume usually employed. In some cases, less than that volume and not pleura surface nodules, it can be really difficult to detect the spot because of its small dimension.
Potential complications of the technique are contrast spreading all around the pleural space, mainly in those cases with subpleural nodules, and pneumothorax in the same cases, both of them checked and solved during the procedure. However, in most studies, no complications were reported (25,26). In our series (11 patients), just one case of pleural spread blue methylene has been encountered.
Main objective of ENB and dye marking is to improve localization during surgical procedure in order to enhance chances of a successful nodule VATS or robotic-assisted thoracic surgery (RATS) resection (avoiding open thoracotomy to touch the lung). Comparing this technique with transthoracic procedures, ENB looks to be less dependent on tumor size (27).
ENB can play an important role in glass ground opacities (GGOs). These sorts of lesions are usually non-palpable or very difficult to palpate. ENB help us identifying them and making them visible. Due to the increase of lung cancer screening programs, small pulmonary nodules are frequently observed (28). Another analysis of 8 large trials of lung cancer screening showed that nodules that were GGOs were more likely to be malignant (59% vs. 73%) than solid nodules (7% to 9%) (29).
On the other hand, as a new technique, there are several premises to check. In intrapulmonary nodules, it is unclear whether to mark in the nodule, next place to visceral pleura or mid-place between pleura and nodule. Neither is it which contrast can achieve better outcomes. These hypotheses should be further investigated.
HOR: the future is here
According to Diana et al. (30) the greatest surgical innovation of the past 30 years is the minimally invasive surgery concept. This new notion is changing not only the way we work but, also, where we work because the technology that comes with this revolution makes structural changes a must.
HOR are rooms that lodge interventional radiology technology and surgical equipment allowing for a combined use of all that technology on one patient in the same place and at the same time. It is a new treatment environment (31). They are larger suites than traditional OR allowing enough room for accommodating the new people and their equipment that will collaborate with the usual inhabitants and equipment of any traditional operating theater. It has been estimated that 8 to 20 people can be working at the same time depending on the procedure (32). On the other hand, these rooms are recommended to be within the surgical area of the hospital when this area is separated from the radiology interventional area (33). Several aspects must be defined when designing a HOR: hygienic requirements, air-flow requirements, type of table, equipment requirements and video solutions among others. As a multifunctional room, the cost of creating a HOR might be significant therefore it should be developed to support a broad variety of procedures. But this economic necessity brings in an interesting problem: the distribution of the different equipment in order to facilitate all the manoeuvers without significantly moving the patient in the transition between interventional and surgical stages of the procedure. To solve this problem, innovations and structural changes had been applied depending on the main activity of the HOR (34).
Imaging is one of the pillars to build a hybrid suite or HOR. This suite should provide multimodality imaging with integrated hardware and software capable of 3D modelling and real-time guidance for interventional and surgical procedures (35). It allows the image-guided VATS (iVATS) an impressive tool for localizing small, non-visible, non-palpable pulmonary nodules. Therefore, any HOR dedicated to thoracic surgery should lodge imaging and navigating technology (36). Normally a cone-beam CT scan (CBCT), also named C-arm CT or Dyna-CT, should be placed by the surgical table and fixed to the ceiling (35-37). For the working teams, the routine use of radiation for diagnostic purposes is a problem and should be under control. The use of protocols for minimizing radiation exposure and individual dosimeters should be available and must be guaranteed (38,39). On the other hand, multiple monitors for displaying previous generated 3D-models of the case, the real-time CT-scan and nodule-marking images and to perform the surgical procedure should be available. These displays can be fixed on the walls, on console areas for radiological manipulation or can be mobile, placed on mobile arms. The possibility to incorporate Bluetooth connections between mobile monitors will eliminate the presence of loose wires on the floor facilitating the mobility of the personnel. The ENB device should have a relevant place. Before its installation, some technical adjustments of the surgical table are needed according to the manufacturer. Also, technicians must ensure that no electromagnetic interference occurs for complete compatibility but, even without interference, only disconnecting the ENB will guarantee the best CT images. Normally a designated position of the device respect to the surgical table is recommended (40).
Although the greatest novelty of the HOR is the integration of imaging and surgery, it does not mean that the surgical procedure cannot be performed using a robot (30,41). Even when nowadays that combination is not very common because of its cost, probably it will in the future.
Surgical technical improvements have come to stay. The cyber surgery or cybernetic surgery (42) is the result of combining developments from the computer science and robotics. Both sciences have a large room for improvement. Our environment will also change to accommodate those innovations and we should move accordingly. Hybrid OR will help us providing better care and working more efficiently for the patient who will be and will feel as the centre of the health care system (43).
Conclusions
The number of patients referred to Thoracic surgery with subcentimetric pulmonary nodules is increasing as part of early diagnosis programs. On the other hand, minimally invasive surgery has proved advantages in terms of pain, complications, length of in-hospital stay..., becoming a first choice. As a consequence, VATS palpation and localization of small pulmonary nodules is really challenging.
Several tools to make nodule identification easier have been discussed. To decide which technique should be preferable, we must take into account availabilities in our institution. As we mentioned before, hook-wire localization is the most commonly employed method. It is performed by a radiologist CT-guided, so it is not very facility-dependent. However, methods like ENB and dye marking followed by thoracoscopic resection, involve better accuracy diminishing inconveniences for patients such as pain and additional procedures.
In those institutions whether a HOR is available, it could help improve accuracy in nodule identification. Whether both ENB and HOR are accomplished together, accuracy and security can even improve.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Precision Cancer Medicine for the series “Precision Surgery for Lung Cancer”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pcm.2019.03.05). The series “Precision Surgery for Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. MFJ serves an unpaid editorial board member of Precision Cancer Medicine from Aug 2018 to Jul 2020 and served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nakashima S, Watanabe A, Obama T, et al. Need for preoperative computed tomography guided localization in video-assisted thoracoscopic surgery pulmonary resections of metastatic pulmonary nodules. Ann Thorac Surg 2010;89:212-8. [Crossref] [PubMed]
- Mack MJ, Gordon MJ, Postma TW, et al. Percutaneous localization of pulmonary nodules for thoracoscopic lung resection. Ann Thorac Surg 1992;53:1123-4. [Crossref] [PubMed]
- Klinkenberg TJ, Dinjens L, Wolf RFE, et al. CT-guided percutaneous hookwire localization increases the efficacy and safety of VATS for pulmonary nodules. J Surg Oncol 2017;115:898-904. [Crossref] [PubMed]
- Chen YR, Yeow KM, Lee JY, et al. CT-guided hook wire localization of subpleural lung lesions for video-assisted thoracoscopic surgery (VATS). J Formos Med Assoc 2007;106:911-8. [Crossref] [PubMed]
- Eichfeld U, Dietrich A, Ott R, et al. Video-assisted thoracoscopic surgery for pulmonary nodules after computed tomography-guided marking with a spiral wire. Ann Thorac Surg 2005;79:313-6; discussion 316-7. [Crossref] [PubMed]
- Dendo S, Kanazawa S, Ando A, et al. Preoperative localization of small pulmonary lesions with a short hook wire and suture system: experience with 168 procedures. Radiology 2002;225:511-8. [Crossref] [PubMed]
- Miyoshi K, Toyooka S, Gobara H, et al. Clinical outcomes of short hook wire and suture marking system in thoracoscopic resection for pulmonary nodules. Eur J Cardiothorac Surg 2009;36:378-82. [Crossref] [PubMed]
- Ciriaco P, Negri G, Puglisi A, et al. Video-assisted thoracoscopic surgery for pulmonary nodules: rationale for preoperative computed tomography-guided hookwire localization. Eur J Cardiothorac Surg 2004;25:429-33. [Crossref] [PubMed]
- Chen S, Zhou J, Zhang J, et al. Video-assisted thoracoscopic solitary pulmonary nodule resection after CT-guided hookwire localization: 43 cases report and literature review. Surg Endosc 2011;25:1723-9. [Crossref] [PubMed]
- Mayo JR, Clifton JC, Powell TI, et al. Lung nodules: CT-guided placement of microcoils to direct video-assisted thoracoscopic surgical resection. Radiology 2009;250:576-85. [Crossref] [PubMed]
- Sancheti MS, Lee R, Ahmed SU, et al. Percutaneous fiducial localization for thoracoscopic wedge resection of small pulmonary nodules. Ann Thorac Surg 2014;97:1914-8; discussion 1919.
- Lizza N, Eucher P, Haxhe JP, et al. Thoracoscopic resection of pulmonary nodules after computed tomographic-guided coil labeling. Ann Thorac Surg 2001;71:986-8. [Crossref] [PubMed]
- Toba H, Kondo K, Miyoshi T, et al. Fluoroscopy-assisted thoracoscopic resection after computed tomography-guided bronchoscopic metallic coil marking for small peripheral pulmonary lesions. Eur J Cardiothorac Surg 2013;44:e126-32. [Crossref] [PubMed]
- Finley RJ, Mayo JR, Grant K, et al. Preoperative computed tomography-guided microcoil localization of small peripheral pulmonary nodules: a prospective randomized controlled trial. J Thorac Cardiovasc Surg ;2015:26-31. [PubMed]
- Miyoshi T, Kondo K, Takizawa H, et al. Fluoroscopy-assisted thoracoscopic resection of pulmonary nodules after computed tomography--guided bronchoscopic metallic coil marking. J Thorac Cardiovasc Surg 2006;131:704-10. [Crossref] [PubMed]
- Moon SW, Wang YP, Jo KH, et al. Fluoroscopy-aided thoracoscopic resection of pulmonary nodule localized with contrast media. Ann Thorac Surg 1999;68:1815-20. [Crossref] [PubMed]
- Watanabe K, Nomori H, Ohtsuka T, et al. Usefulness and complications of computed tomography-guided lipiodol marking for fluoroscopy-assisted thoracoscopic resection of small pulmonary nodules: experience with 174 nodules. J Thorac Cardiovasc Surg 2006;132:320-4. [Crossref] [PubMed]
- Mogi A, Yajima T, Tomizawa K, et al. Video-assisted thoracoscopic surgery after preoperative CT-guided lipiodol marking of small or impalpable pulmonary nodules. Ann Thorac Cardiovasc Surg 2015;21:435-9. [Crossref] [PubMed]
- Chella A, Lucchi M, Ambrogi MC, et al. A pilot study of the role of TC-99 radionuclide in localization of pulmonary nodular lesions for thoracoscopic resection. Eur J Cardiothorac Surg 2000;18:17-21. [Crossref] [PubMed]
- Galetta D, Bellomi M, Grana C, et al. Radio-Guided Localization and Resection of Small or Ill-Defined Pulmonary Lesions. Ann Thorac Surg 2015;100:1175-80. [Crossref] [PubMed]
- Ambrogi MC, Melfi F, Zirafa C, et al. Radio-guided thoracoscopic surgery (RGTS) of small pulmonary nodules. Surg Endosc 2012;26:914-9. [Crossref] [PubMed]
- Park CH, Han K, Hur J, et al. Comparative Effectiveness and Safety of Preoperative Lung Localization for Pulmonary Nodules. A Systematic Review and Meta-analysis. Chest 2017;151:316-28. [Crossref] [PubMed]
- Awais O, Reidy MR, Mehta K, et al. Electromagnetic Navigation Bronchoscopy-Guided Dye Marking for Thoracoscopic Resection of Pulmonary Nodules. Ann Thorac Surg 2016;102:223-9. [Crossref] [PubMed]
- Muñoz-Largacha JA, Ebright MI, Litle VR, et al. Electromagnetic navigational bronchoscopy with dye marking for identification of small peripheral lung nodules during minimally invasive surgical resection. J Thorac Dis 2017;9:802-8. [Crossref] [PubMed]
- Krimsky WS, Minnich DJ, Cattaneo SM, et al. Thoracoscopic detection of occult indeterminate pulmonary nodules using bronchoscopic pleural dye marking. J Community Hosp Intern Med Perspect 2014;4: [Crossref] [PubMed]
- Patrucco F, Gavelli F, Daverio M, et al. Electromagnetic Navigation Bronchoscopy: Where Are We Now? Five Years of a Single-Center Experience. Lung 2018;196:721-7. [Crossref] [PubMed]
- Bolton WD, Howe H, Stephenson JE. The Utility of Electromagnetic Navigational Bronchoscopy as a Localization Tool for Robotic Resection of Small Pulmonary Nodules. Ann Thorac Surg 2014;98:471-5; discussion 475-6. [Crossref] [PubMed]
- Yankelevitz DF, Yip R, Smith JP, et al. CT Screening for Lung Cancer: Nonsolid Nodules in Baseline and Annual Repeat Rounds. Radiology 2015;277:555-64. [Crossref] [PubMed]
- Wahidi MM, Govert JA, Goudar RK, et al. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:94S-107S.
- Diana M, Marescaux J. Robotic surgery. Br J Surg 2015;102:e15-28. [Crossref] [PubMed]
- Mansour MA. The new operating room environment. Surg Clin North Am 1999;79:477-87. [Crossref] [PubMed]
- ten Cate G, Fosse E, Hol PK, et al. Integrating surgery and radiology in one suite: a multicenter study. J Vasc Surg 2004;40:494-9. [Crossref] [PubMed]
- Bonatti J, Vassiliades T, Nifong W, et al. How to build a cath-lab operating room. Heart Surg Forum 2007;10:E344-8. [Crossref] [PubMed]
- Ashour R, See AP, Dasenbrock HH, et al. Refinement of the Hybrid Neuroendovascular Operating Suite: Current and Future Applications. World Neurosurg 2016;91:6-11. [Crossref] [PubMed]
- Gill RR, Zheng Y, Barlow JS, et al. Image-guided video assisted thoracoscopic surgery (iVATS) - phase I-II clinical trial: Image-guided Video Assisted Thoracoscopic Surgery (iVATS). J Surg Oncol 2015;112:18-25. [Crossref] [PubMed]
- Ujiie H, Effat A, Yasufuku K. Image-guided thoracic surgery in the hybrid operation room. J Vis Surg 2017;3:148. [Crossref] [PubMed]
- Zhao ZR, Lau RW, Ng CS. Hybrid theatre and alternative localization techniques in conventional and single-port video-assisted thoracoscopic surgery. J Thorac Dis 2016;8:S319-27. [PubMed]
- Schuetze K, Kraus M, Eickhoff A, et al. Correction to: Radiation exposure for intraoperative 3D scans in a hybrid operating room: how to reduce radiation exposure for the surgical team. Int J Comput Assist Radiol Surg 2018;13:1683. [Crossref] [PubMed]
- Schuetze K, Kraus M, Eickhoff A, et al. Radiation exposure for intraoperative 3D scans in a hybrid operating room: how to reduce radiation exposure for the surgical team. Int J Comput Assist Radiol Surg 2018;13:1291-300. [Crossref] [PubMed]
- Ng CS, Yu SC, Lau RW, et al. Hybrid DynaCT-guided electromagnetic navigational bronchoscopic biopsy†. Eur J Cardiothorac Surg 2016;49:i87-8. [PubMed]
- Tsuda S, Oleynikov D, Gould J, et al. SAGES TAVAC safety and effectiveness analysis: da Vinci® Surgical System (Intuitive Surgical, Sunnyvale, CA). Surg Endosc 2015;29:2873-84. [Crossref] [PubMed]
- Marescaux J, Diana M. Cybersurgery: human-machine integration for surgery of the future. Bull Acad Natl Med 2013;197:1291-301. [PubMed]
- Hirsch R. The hybrid cardiac catheterization laboratory for congenital heart disease: From conception to completion. Catheter Cardiovasc Interv 2008;71:418-28. [Crossref] [PubMed]
Cite this article as: Jimenez MF, Fuentes MG, Gomez-Hernandez MT, Novoa NM. Planning and marking small nodules for surgery. Precis Cancer Med 2019;2:11.