
Microsatellite instability and immunotherapy in gastric cancer: a narrative review
Introduction
Gastric cancer remains one of the most common cancer worldwide and is responsible for more than one million new cases and an estimated 769,000 deaths in 2020, ranking fifth in cancer incidence and fourth in cancer mortality (1). Surgical resection with adjuvant chemotherapy is widely acknowledged as an effective treatment for the early-stage gastric cancer. Surgery followed by chemotherapy may be an approach to treating localized gastric cancer, while in other parts of the world, neoadjuvant chemotherapy followed by surgery and adjuvant chemotherapy are also other approaches to gastric cancer treatment. In general, systemic therapy and chemoradiotherapy remain the standard first-line treatments in locally advanced or metastatic gastric cancer cases (2). Nevertheless, despite the advances of current comprehensive treatments, the 5-year survival rate remains very low, and a tremendous effort is still required for individualized treatment of gastric cancer and the improvement of clinical prognosis.
Gastric cancer is highly heterogeneous, and The Cancer Genome Atlas (TCGA) Research Network has classified gastric cancer into four subtypes based on a comprehensive molecular characterization: chromosome instability (CIN), microsatellite instability (MSI), genomically stable (GS), and Epstein-Barr virus (EBV) infected type (3). In addition, the Asian Cancer Research Group (ACRG) also proposes a novel gastric cancer molecular classification, identifying four molecular subtypes: MSI type, microsatellite stable with epithelial to mesenchymal transition features (MSS/EMT), MSS with tumor protein p53 (TP53)-active (MSS/TP53+) type and MSS with TP53-inactive (MSS/TP53−) type (4). Interestingly, both TCGA and ACRG researches distinguish the MSI entity as a separate and well-defined gastric cancer subgroup.
Tumor molecular classifications based on the comprehensive molecular profiles have more clinical significance in predicting treatment efficacy than traditional Lauren’s classification and World Health Organization (WHO) classification, especially for immunotherapy (3,5). Over the past few years, immunotherapy has received great attention in cancer treatment, and has been proven to greatly improve the therapeutic effect and survival of gastric cancer. Nevertheless, the widespread clinical application of immunotherapy has been limited owing to the relative poor efficacy and low clinical response rates (2). Therefore, the specific biomarkers are desperately required to discriminate responders from non-responders. An increasing evidence suggests that MSI status is associated with the response to immunotherapy in advanced gastric cancer (6,7). In addition, a relative new development in the workflow of all newly diagnosed gastric cancer patients is the recommendation of MSI detection in National Comprehensive Cancer Network (NCCN) clinical guidelines (8). Meanwhile, the application of immune checkpoint blockade have been granted the US Food and Drug Administration (FDA) approval for deficient mismatch repair (dMMR) and MSI-high (MSI-H) solid tumors (9). MSI may serve as a biomarker of immunotherapy for gastric cancer. However, the correlation between MSI and clinicopathological features in gastric cancer is still unclear.
In this review, we analyze the current evidence about MSI-H gastric cancer from a clinical perspective, focusing on the molecular and pathological features, prognostic values, and the future perspectives for immunotherapeutic applications in the MSI-H gastric cancer subgroup. We now present the subsequent article according to the Narrative Review reporting checklist (available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-48/rc).
Methods
In this narrative review, we focused on MSI and immunotherapy in gastric cancer. We performed a systematic literature research on PubMed from January 11, 2017 to August 12, 2022, using the keywords: “MSI”, “immunotherapy”, and “gastric cancer”. We selected articles published in the English language (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | From January 11, 2017 to August 12, 2022 |
| Databases and other sources searched | PubMed |
| Search terms used | Microsatellite instability, immunotherapy, and gastric cancer |
| Timeframe | Up to August 12, 2022 |
| Inclusion and exclusion criteria | We only included studies published in English language |
| Selection process | The selection process was conducted independently by the authors |
MSI and MMR system
Those tumor individuals with MSI-H or dMMR status exhibit a favorable response to immunotherapy (10). Microsatellites are short tandem repeat DNA sequences of mononucleotide, or higher-order nucleotide repeats, which are located throughout the entire human genome (11). MSI refers to a hypermutator phenotype that happens at genomic microsatellites coupled with a dMMR machinery (12). Due to the repeated structures, microsatellites are specially vulnerable to replication errors that are usually repaired by the MMR machinery (13). MMR machinery was initially discovered as a specific somatic instability in Lynch syndrome and was subsequently identified as a microsatellite (14,15). MMR is an extremely conserved cellular process, including a specific set of MMR genes. Under the normal DNA replication conditions, DNA mismatch sites are originally recruited and combined with mutS homolog (MSH)2/MSH6 heterodimers, in turn, mutL homolog 1 (MLH1)/PMS1 homolog 2 (PMS2) complex is responsible for the precise excision and synthesis of a corrected strand to replace the mismatched sites. Both abnormal expressions and defects in MMR elements lead to deficiency of MMR system and subsequent failed repair of mismatched DNA sites; this phenotype is known as MSI (Figure 1) (16).
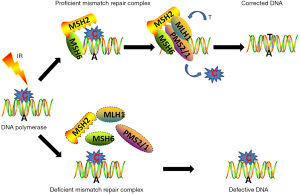
Mutations in MMR genes result in an accumulation of DNA replication errors, which further leads to MSI (12). Indeed, the abnormal MMR genes are proved to be the transforming events to determine the tumor progression (17). Due to the large amount of microsatellites and spread over the entire genome, the MSI will lead to the dysfunction of multiple genes in numerous signaling pathways associated with tumors, further leading to the development of MSI-H tumors (18). In addition, MSI-H cancers harbor a maximum of 1,000-fold elevated mutation frequencies of missense mutations compared with MSS malignancies (19,20). Mutations occur frequently in DNA repair genes MRE11A and hRAD50, kinase genes BRAF and PIK3CA, and MMR genes MSH3 and MSH6, leading to the abnormal cellular functions and signaling pathways (13).
MSI gastric cancer
Evidences in the literatures (3,21) reported the MSI-H rates in gastric cancer patients (about 5–22%) generally depended on the geographical differences, the different tumor stages and the approaches utilized to analyze the MSI status (22). MSI gastric cancer is related to an older age, tumoral location, lack of lymph node metastases and lower malignant potential (23). Additionally, MSI gastric cancers are more inclined to be diagnosed at an earlier tumor stage and categorized as the intestinal Lauren’s histological type (4,23,24). Since MSI mainly occurs at the early stage of tumorigenesis, some studies have demonstrated the occurrence of MSI in most gastric precancerous lesions, demonstrating that MSI may be served as an early event during gastric tumorigenesis (25,26). A previous multinational meta-analysis showed that MSI status could be served as a robust prognostic marker in patients with resectable primary gastric cancer (27). Compared with the MSS/MSI-low (MSI-L) subgroup, MSI-H status group demonstrated a better outcome in surgery-alone group [hazard ratio (HR), 0.35; 95% confidence interval (CI): 0.11 to 1.11; P=0.08] and a worse survival prognosis in chemotherapy + surgery treatment group (HR, 2.22; 95% CI: 1.02 to 4.85; P=0.04] in MAGIC trial (28). In addition, the methylation accumulation of MMR genes during gastric cancer progression has been also reported. Epigenetic silence of hMLH1 caused by promoter hypermethylation accounts for primary reason of dMMR during progression of gastric cancer, however, the mutations in hMLH1 and hMSH2 genes are less common (29-31). hMLH1 gene mutations and methylation are mainly correlated with immunohistochemistry (IHC) loss of MLH1 and PMS2 proteins. Over 50% of MSI-H gastric cancer subgroup contain hypermethylation of hMLH1 promoter, while mutations in hMLH1 are present in approximately 15% of MSI-H gastric cancers (17).
Studies have explored the molecular features of MSI gastric cancer, and identified a series of distinctive changed genes in the unique molecular subtype (3,4). Thirty-seven genes are significantly mutated in MSI gastric cancer according to the whole-genome analysis of TCGA dataset. These altered genes participate in a series of cellular life processes including the regulation of cell cycle, DNA integrity maintenance, chromatin remodeling, cell death, transcription regulation, apoptosis regulation and cell signal transduction. MSI gastric cancer also shows increased expressions of multiple mitotic network moleculars (3,4). KRAS mutation is significantly associated with MSI status (32-34). A recent research conducted on 595 gastric cancer patients, examining the KRAS mutation in 14.9% of MSI cases, and 1.2% of MSS. And so beyond that, patients with both KRAS mutation and MSI demonstrated a better prognosis compared with KRAS mutation and MSS patients (33,35). Furthermore, another large multicenter study was performed to detect the KRAS mutation and MMR status in locally advanced resectable gastric cancer, and the observations also confirmed the significant association between the KRAS mutation and dMMR status (36).
MSI detection
Cancers harboring a dMMR mechanism are frequently hypermutated in monomorphic microsatellites that are extremely inclined to mismatch errors. The context is defined as MSI, which can be usually detected by IHC and two other molecular examinations, including conventional MSI polymerase chain reaction (PCR) and the new next-generation sequencing (NGS) approaches (37,38).
IHC for MMR proteins is used as the first-line approach for the MSI detection due to the convenience of testing and less measurement criteria of tumor tissue compared with other molecular tests (37). MLH1, MSH6, and PMS2 antibodies for the determination of MMR proteins are commonly used, and the explanation of the findings is dependent upon the biological roles of the complex created by the detected genes (39). In fact, the alterations in the MMR genes are also in charge of protein degradation of specific complex. Mutations in MSH2 are generally correlated to the IHC deficiency in MSH2 and MSH6 proteins (37,40). Therefore, IHC detection leads to imperfect MMR genes testing and requires further detection of MMR genetic analysis.
PCR amplification with specific primers for microsatellite repeats results in a unique magnification curve. According to the length of nucleotide repeats in tumor and adjacent normal mucosa, MSI can be evaluated as a “shift” in the phenograms of one or more microsatellites (41). Usually, two mononucleotides (BAT25 and BAT26) and three dinucleotides (D5S346, D2S123, and D17S250) are routine testing sites in Bethesda panel for the MSI molecular detection (16,42). These regions are amplified using fluorescent multiplex PCR and the amplification products are further detected by the following capillary electrophoresis (43). If two or more loci (or >30% of loci) are found, the tumor is regarded as MSI-H; if only one locus (or in 10–30% of loci) is detected, the tumor is defined as MSI-L; MSS, indicating none of the markers (or <10% of loci) with instability (13,44). MSI-H and dMMR are highly concordant in many cancers, and usually these two terms can be used in place of each other (45). The variability of MSI-H frequency varies in different tumor types, among which uterine corpus cancer, colon cancer, and gastric cancer rank in the top three malignancies (46,47).
In 2014, NGS with whole genome sequencing was firstly suggested as an alternative tool for the verification of the MSI phenotypes (48,49). The superiority of NGS analysis for MSI assessment is that it is not tumor-type-specific, and it does not need the matched normal specimen. Moreover, NGS-based method covers a wider range of microsatellite sites, allowing it not restricted to the conventional microsatellite loci detected by PCR/IHC-based approaches (50). However, the high expenses for NGS and the expertise required to analyze the NGS data limit the widespread application of NGS in the routine clinical diagnosis.
Immunotherapy for MSI gastric cancer
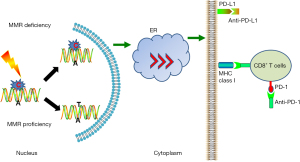
Previous clinical trials manifested that dMMR or MSI status were obviously associated with the response to the immune checkpoint inhibitors (ICIs), independent of the sites of tumor origin (47,51,52). MSI-H status has been put forward as a positive indicator of ICIs efficacy in advanced cancer patients. Evidence for the application of immunotherapy in MSI-H gastric cancer comes from the specific hypermutated phenotype in the subgroup (7). Meanwhile, MSI-H gastric cancers are able to express plentiful peptides that can trigger tumor infiltrating lymphocytes (TIL) recruitment and activation (27,53). The effector T cells in tumor microenvironment (TME) and T-cell exhaustion status are proved to be significantly associated with the response to pembrolizumab in MSI gastric cancer, and this means that both quantity and functional status of TIL in the TME are indispensable (27). In the KEYNOTE-012 trial (54), 22% of programmed death-ligand 1 (PD-L1)-positive advanced gastric cancer patients obtained an overall response. Further genome analysis showed MSI-H in 17% of all enrolled patients, and half of the MSI-H patients had a partial response. In addition, MSI-H tumors demonstrated responses to ICIs independent of the PD-L1 expression (55,56). These observations from all above studies proposed evidences for potential application of MSI as a predictor for immunotherapy (57,58). In another encouraging KEYNOTE-059 clinical trial, the therapeutic effect of pembrolizumab was also evaluated in gastric/gastroesophageal junction cancer patients. Of note, patients with MSI demonstrated an objective response rate (ORR) of 57.1%, while the patients with MSS exhibited a lower ORR (9%) (59). Among the MSI-H patients recruited in KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 clinical trials, higher response rates to pembrolizumab immunotherapy were 57.1%, 46.7%, and 57.1%, respectively. However, the median overall survival (OS) was not reached (NR) for pembrolizumab among MSI-H patients. Based on these findings, FDA approved the approval for pembrolizumab application in PD-L1 positive metastatic gastric cancer patients and unresectable dMMR/MSI solid tumor patients, independent of the primary cancer (58). In addition, the phase II KEYNOTE-158 trial also validated a significant curative effect of pembrolizumab for gastric cancer individuals with nonresponsive in the traditional standard medications. MSI-H gastric cancer patients presented an ORR of 46% and a PFS of 11 months (60). Another clinical trial worthy of being analyzed is CHECKMATE-032 trial, which was performed to explore the efficacy and safety of nivolumab in PD-L1 unselected metastatic gastric cancer patients. The 12-month OS rates of MSI-H patients were 57% in the NIVO3 group, 50% in the NIVO1-plus-IPI3 group, and 50% in the NIVO3-plus-IPI1 group, while the OS rates of the patients with non-MSI-H patients were 33%, 32%, and 23%, respectively (61). Subgroup analysis revealed that MSI patients achieved a better median OS compared with the MSS entirety.
As to post-hoc analyses of MSI-H predictive effect in randomized controlled trials (RCTs), including the KEYNOTE-061, KEYNOTE-062, JAVELIN Gastric 100 (62), and CHECKMATE-649 phase III trials, a meta-analysis was performed and covered a total of 2,545 advanced gastric cancer patients. In total, 4.8% of the recruited participants displayed MSI-H status, and demonstrated an HR for OS benefit of 0.34 when applied with anti-programmed death 1 (PD-1) drugs versus chemotherapy. These results promote a better efficacy of pembrolizumab than the chemotherapy in support of the median OS (63).
Although these findings acquired in aforementioned studies are tremendously encouraging, due to the relatively few recruited participants and the low phenotypic frequency of MSI subtype gastric cancer, the application of ICIs in MSI patients is obviously not as good as the colorectal cancer trials findings (64). Some representative clinical trials on ICIs for MSI gastric cancer are listed below (Table 2). Nevertheless, immunotherapy opens up a new way of cancer treatment and improves the therapeutic effect of MSI gastric cancer, emphasizing a strong theoretical support for the management of ICIs in MSI subtype (Figure 2).
Table 2
| Reference | ClinicalTrials.gov number | Phase | Tumor type | Treatment settings [number of participants] | Results |
|---|---|---|---|---|---|
| Kwon et al. (27) | NCT02589496 | II | MSI-H gastric cancer | Pembrolizumab [61] | ORR of 55.6% and DCR of 88.9% |
| Muro et al. (54) | KEYNOTE-012 (NCT01848834) | Ib | PD-L1+ advanced gastric cancer | Pembrolizumab [39] | MSI gastric cancer ORR 50% |
| Fuchs et al. (59) | KEYNOTE-059 (NCT02335411) | II | G/GEJ cancer | Pembrolizumab [259] | MSI gastric cancer ORR of 57.1% |
| Shitara et al. (65) | KEYNOTE-061 (NCT02370498) | III | G/GEJ cancer | Pembrolizumab [296] | MSI gastric cancer ORR of 46.7% |
| Shitara et al. (66) | KEYNOTE-062 (NCT02494583) | III | G/GEJ cancer | Pembrolizumab [256]; pembrolizumab plus chemotherapy [257]; chemotherapy [250] | MSI gastric cancer ORR of 57.1% |
| Marabelle et al. (60) | KEYNOTE-158 (NCT02628067) | II | Nonresponsive gastric cancer | Pembrolizumab [163] | MSI gastric cancer ORR of 45.8% PFS, 11.0 months |
| Janjigian et al. (61) | CHECKMATE-032 (NCT02267343) | I/II | PD-L1 unselected metastatic gastric cancer | Nivolumab 3 mg/kg [59]; nivolumab 1 mg/kg plus ipilimumab 3 mg/kg [49]; nivolumab 3 mg/kg plus ipilimumab 1 mg/kg [52] | MSI gastric cancer OS 15 months |
| Janjigian et al. (67) | CHECKMATE-649 (NCT02872116) | III | Gastric, gastroesophageal junction, or esophageal adenocarcinoma | Nivolumab plus chemotherapy [789]; chemotherapy [792] | Nivolumab plus chemotherapy OS (HR: 0.71) and PFS (HR: 0.68) |
ICIs, immune checkpoint inhibitors; MSI, microsatellite instability; MSI-H, MSI-high; ORR, objective response rate; DCR, disease control rate; PD-L1, programmed death-ligand 1; G/GEJ, gastric/gastroesophageal junction; PFS, progression-free survival; OS, overall survival; HR, hazard ratio.
Conclusions
Although the efficacy of surgical treatment and targeted therapy have been greatly improved, gastric cancer remains one of the most important global disease burdens. The complexity of gastric cancer put forward higher demands for novel molecular-based individual therapeutics. The comprehensive classification of gastric cancer into four well-defined molecular subtypes, laying the foundation for propose innovative treatment strategies for the patients with specific molecular features. MSI gastric cancers take up a relatively small patients population, and show distinctive clinicopathological features. The favorable prognosis resulting from the MSI cancers treated with ICIs should be taken into account in the future clinical practice. Despite the retrospective feature of the clinical researches generally included for analysis, and the relative small number of MSI gastric cancer patients recruited in most clinical trials, the purpose of our current review is to illustrate the MSI entity as a specific subtype and these patients may be prone to immunotherapy. In conclusion, MSI detection has a promising role in guiding the immunotherapy for gastric cancer. However, they still need to be further verified in larger prospective trials for this specific clinical type.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-48/rc
Peer Review File: Available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-48/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-48/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin 2021;71:264-79. [Crossref] [PubMed]
- Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9. [Crossref] [PubMed]
- Cristescu R, Lee J, Nebozhyn M, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med 2015;21:449-56. [Crossref] [PubMed]
- Li X, Zhang L, Wang C, et al. Microsatellite instability in Chinese gastric cancer and its correlation with clinical characteristics. J Gastrointest Oncol 2021;12:2719-27. [Crossref] [PubMed]
- Jain R, Denlinger CS, Dotan E. Refining Immunotherapy for the Treatment of Gastric Cancer With High Microsatellite Instability. JAMA Oncol 2021;7:902-3. [Crossref] [PubMed]
- Chao J, Fuchs CS, Shitara K, et al. Assessment of Pembrolizumab Therapy for the Treatment of Microsatellite Instability-High Gastric or Gastroesophageal Junction Cancer Among Patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 Clinical Trials. JAMA Oncol 2021;7:895-902. [Crossref] [PubMed]
- Ajani JA, D'Amico TA, Bentrem DJ, et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022;20:167-92. [Crossref] [PubMed]
- Lemery S, Keegan P, Pazdur R. First FDA Approval Agnostic of Cancer Site - When a Biomarker Defines the Indication. N Engl J Med 2017;377:1409-12. [Crossref] [PubMed]
- Yamamoto H, Imai K. Microsatellite instability: an update. Arch Toxicol 2015;89:899-921. [Crossref] [PubMed]
- Findeisen P, Kloor M, Merx S, et al. T25 repeat in the 3' untranslated region of the CASP2 gene: a sensitive and specific marker for microsatellite instability in colorectal cancer. Cancer Res 2005;65:8072-8. [Crossref] [PubMed]
- Baretti M, Le DT. DNA mismatch repair in cancer. Pharmacol Ther 2018;189:45-62. [Crossref] [PubMed]
- Vilar E, Gruber SB. Microsatellite instability in colorectal cancer-the stable evidence. Nat Rev Clin Oncol 2010;7:153-62. [Crossref] [PubMed]
- Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science 1993;260:816-9. [Crossref] [PubMed]
- Ionov Y, Peinado MA, Malkhosyan S, et al. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 1993;363:558-61. [Crossref] [PubMed]
- Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology 2010;138:2073-87.e3. [Crossref] [PubMed]
- Ratti M, Lampis A, Hahne JC, et al. Microsatellite instability in gastric cancer: molecular bases, clinical perspectives, and new treatment approaches. Cell Mol Life Sci 2018;75:4151-62. [Crossref] [PubMed]
- Jiricny J. The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol 2006;7:335-46. [Crossref] [PubMed]
- Corso G, Velho S, Paredes J, et al. Oncogenic mutations in gastric cancer with microsatellite instability. Eur J Cancer 2011;47:443-51. [Crossref] [PubMed]
- Dudley JC, Lin MT, Le DT, et al. Microsatellite Instability as a Biomarker for PD-1 Blockade. Clin Cancer Res 2016;22:813-20. [Crossref] [PubMed]
- Kim JY, Shin NR, Kim A, et al. Microsatellite instability status in gastric cancer: a reappraisal of its clinical significance and relationship with mucin phenotypes. Korean J Pathol 2013;47:28-35. [Crossref] [PubMed]
- De' Angelis GL. Microsatellite instability in colorectal cancer. Acta Biomed 2018;89:97-101. [PubMed]
- Polom K, Marano L, Marrelli D, et al. Meta-analysis of microsatellite instability in relation to clinicopathological characteristics and overall survival in gastric cancer. Br J Surg 2018;105:159-67. [Crossref] [PubMed]
- Martinez-Ciarpaglini C, Fleitas-Kanonnikoff T, Gambardella V, et al. Assessing molecular subtypes of gastric cancer: microsatellite unstable and Epstein-Barr virus subtypes. Methods for detection and clinical and pathological implications. ESMO Open 2019;4:e000470. [Crossref] [PubMed]
- Li B, Liu HY, Guo SH, et al. Microsatellite instability of gastric cancer and precancerous lesions. Int J Clin Exp Med 2015;8:21138-44. [PubMed]
- Sugimoto R, Sugai T, Habano W, et al. Clinicopathological and molecular alterations in early gastric cancers with the microsatellite instability-high phenotype. Int J Cancer 2016;138:1689-97. [Crossref] [PubMed]
- Kwon M, An M, Klempner SJ, et al. Determinants of Response and Intrinsic Resistance to PD-1 Blockade in Microsatellite Instability-High Gastric Cancer. Cancer Discov 2021;11:2168-85. [Crossref] [PubMed]
- Smyth EC, Wotherspoon A, Peckitt C, et al. Mismatch Repair Deficiency, Microsatellite Instability, and Survival: An Exploratory Analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) Trial. JAMA Oncol 2017;3:1197-203. [Crossref] [PubMed]
- Wu MS, Sheu JC, Shun CT, et al. Infrequent hMSH2 mutations in sporadic gastric adenocarcinoma with microsatellite instability. Cancer Lett 1997;112:161-6. [Crossref] [PubMed]
- Bevilacqua RA, Simpson AJ. Methylation of the hMLH1 promoter but no hMLH1 mutations in sporadic gastric carcinomas with high-level microsatellite instability. Int J Cancer 2000;87:200-3. [Crossref] [PubMed]
- Ling ZQ, Tanaka A, Li P, et al. Microsatellite instability with promoter methylation and silencing of hMLH1 can regionally occur during progression of gastric carcinoma. Cancer Lett 2010;297:244-51. [Crossref] [PubMed]
- Brennetot C, Duval A, Hamelin R, et al. Frequent Ki-ras mutations in gastric tumors of the MSI phenotype. Gastroenterology 2003;125:1282. [Crossref] [PubMed]
- Polom K, Das K, Marrelli D, et al. KRAS Mutation in Gastric Cancer and Prognostication Associated with Microsatellite Instability Status. Pathol Oncol Res 2019;25:333-40. [Crossref] [PubMed]
- Arai T, Matsuda Y, Aida J, et al. Solid-type poorly differentiated adenocarcinoma of the stomach: clinicopathological and molecular characteristics and histogenesis. Gastric Cancer 2019;22:314-22. [Crossref] [PubMed]
- Queirós P, Pinheiro H, Carvalho J, et al. KRAS mutations in microsatellite instable gastric tumours: impact of targeted treatment and intratumoural heterogeneity. Virchows Arch 2015;467:383-92. [Crossref] [PubMed]
- van Grieken NC, Aoyama T, Chambers PA, et al. KRAS and BRAF mutations are rare and related to DNA mismatch repair deficiency in gastric cancer from the East and the West: results from a large international multicentre study. Br J Cancer 2013;108:1495-501. [Crossref] [PubMed]
- Luchini C, Bibeau F, Ligtenberg MJL, et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol 2019;30:1232-43. [Crossref] [PubMed]
- Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut 2010;59:666-89. [Crossref] [PubMed]
- Baudhuin LM, Burgart LJ, Leontovich O, et al. Use of microsatellite instability and immunohistochemistry testing for the identification of individuals at risk for Lynch syndrome. Fam Cancer 2005;4:255-65. [Crossref] [PubMed]
- Leite M, Corso G, Sousa S, et al. MSI phenotype and MMR alterations in familial and sporadic gastric cancer. Int J Cancer 2011;128:1606-13. [Crossref] [PubMed]
- Bacher JW, Flanagan LA, Smalley RL, et al. Development of a fluorescent multiplex assay for detection of MSI-High tumors. Dis Markers 2004;20:237-50. [Crossref] [PubMed]
- Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med 2003;348:919-32. [Crossref] [PubMed]
- Berg KD, Glaser CL, Thompson RE, et al. Detection of microsatellite instability by fluorescence multiplex polymerase chain reaction. J Mol Diagn 2000;2:20-8. [Crossref] [PubMed]
- Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 1998;58:5248-57. [PubMed]
- Cicek MS, Lindor NM, Gallinger S, et al. Quality assessment and correlation of microsatellite instability and immunohistochemical markers among population- and clinic-based colorectal tumors results from the Colon Cancer Family Registry. J Mol Diagn 2011;13:271-81. [Crossref] [PubMed]
- Hause RJ, Pritchard CC, Shendure J, et al. Classification and characterization of microsatellite instability across 18 cancer types. Nat Med 2016;22:1342-50. [Crossref] [PubMed]
- Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409-13. [Crossref] [PubMed]
- Salipante SJ, Scroggins SM, Hampel HL, et al. Microsatellite instability detection by next generation sequencing. Clin Chem 2014;60:1192-9. [Crossref] [PubMed]
- Woerner SM, Yuan YP, Benner A, et al. SelTarbase, a database of human mononucleotide-microsatellite mutations and their potential impact to tumorigenesis and immunology. Nucleic Acids Res 2010;38:D682-9. [Crossref] [PubMed]
- Vanderwalde A, Spetzler D, Xiao N, et al. Microsatellite instability status determined by next-generation sequencing and compared with PD-L1 and tumor mutational burden in 11,348 patients. Cancer Med 2018;7:746-56. [Crossref] [PubMed]
- Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib Plus Pembrolizumab in Patients With Advanced Endometrial Cancer. J Clin Oncol 2020;38:2981-92. [Crossref] [PubMed]
- Kole C, Charalampakis N, Tsakatikas S, et al. Immunotherapy for gastric cancer: a 2021 update. Immunotherapy 2022;14:41-64. [Crossref] [PubMed]
- Cho J, Chang YH, Heo YJ, et al. Four distinct immune microenvironment subtypes in gastric adenocarcinoma with special reference to microsatellite instability. ESMO Open 2018;3:e000326. [Crossref] [PubMed]
- Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol 2016;17:717-26. [Crossref] [PubMed]
- Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390:2461-71. [Crossref] [PubMed]
- Ott PA, Le DT, Kim JW, et al. Nivolumab (NIVO) in patients (pts) with advanced (adv) chemotherapy-refractory (CT-Rx) esophagogastric (EG) cancer according to microsatellite instability (MSI) status: checkmate 032. Ann Oncol 2017;28:v229-30. [Crossref]
- Janjigian YY, Bendell JC, Calvo E, et al. CheckMate-032: Phase I/II, open-label study of safety and activity of nivolumab (nivo) alone or with ipilimumab (ipi) in advanced and metastatic (A/M) gastric cancer (GC). J Clin Oncol 2016;34:abstr 4010.
- Fashoyin-Aje L, Donoghue M, Chen H, et al. FDA Approval Summary: Pembrolizumab for Recurrent Locally Advanced or Metastatic Gastric or Gastroesophageal Junction Adenocarcinoma Expressing PD-L1. Oncologist 2019;24:103-9. [Crossref] [PubMed]
- Fuchs CS, Doi T, Jang RW, et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol 2018;4:e180013. [Crossref] [PubMed]
- Marabelle A, Le DT, Ascierto PA, et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol 2020;38:1-10. [Crossref] [PubMed]
- Janjigian YY, Bendell J, Calvo E, et al. CheckMate-032 Study: Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Patients With Metastatic Esophagogastric Cancer. J Clin Oncol 2018;36:2836-44. [Crossref] [PubMed]
- Moehler M, Dvorkin M, Boku N, et al. Phase III Trial of Avelumab Maintenance After First-Line Induction Chemotherapy Versus Continuation of Chemotherapy in Patients With Gastric Cancers: Results From JAVELIN Gastric 100. J Clin Oncol 2021;39:966-77. [Crossref] [PubMed]
- Pietrantonio F, Randon G, Di Bartolomeo M, et al. Predictive role of microsatellite instability for PD-1 blockade in patients with advanced gastric cancer: a meta-analysis of randomized clinical trials. ESMO Open 2021;6:100036. [Crossref] [PubMed]
- Golshani G, Zhang Y. Advances in immunotherapy for colorectal cancer: a review. Therap Adv Gastroenterol 2020;13:1756284820917527. [Crossref] [PubMed]
- Shitara K, Özgüroğlu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 2018;392:123-33. [Crossref] [PubMed]
- Shitara K, Van Cutsem E, Bang YJ, et al. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol 2020;6:1571-80. [Crossref] [PubMed]
- Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021;398:27-40. [Crossref] [PubMed]
Cite this article as: Duan Y, Xu D. Microsatellite instability and immunotherapy in gastric cancer: a narrative review. Precis Cancer Med 2023;6:14.

