
Gonadotropin releasing hormone agonist-related meningioma progression causing abducens nerve palsy: an oncological case report
Introduction
Androgen-deprivation therapy (ADT) is an important component of prostate cancer treatment, either as an adjuvant therapy in intermediate to high-risk localised disease, or for the treatment of metastatic or locally advanced/recurrent prostate cancer (1,2). Gonadotropin releasing hormone (GnRH) agonists are a commonly-prescribed form of ADT. Side-effects of ADT include hot flushes, muscle weakness, osteoporosis, hypertension and non-infective hepatitis (2). GnRH agonist use for prostate cancer has been associated with stimulation of the growth of meningiomas (2-6), which are the most common intracerebral tumours and commonly harbour various hormone receptors, including those for GnRH, progesterone and oestrogen (3,7,8).
Here we describe the development of a reversible abducens nerve palsy in a patient with prostate cancer, treated with a GnRH agonist, whose subsequent cerebral MRI showed a likely ipsilateral cavernous sinus meningioma, enlargement of which presumably caused the abducens nerve compression and palsy. The nerve palsy gradually resolved three months after the GnRH agonist was ceased. We present the following case in accordance with the CARE reporting checklist (available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-57/rc).
Case presentation
In January 2018, a 67-year-old man was investigated for a raised prostate specific antigen (PSA) level of 11.7 ng/mL. The PSA analysis had been performed as part of a routine health check. Rectal examination revealed a stage B1 nodule in his right prostatic apex. He underwent ultrasound-guided prostatic biopsies, which were positive in four of six prostate areas, with a Gleason histopathological score of at least 4+4=8 out of 10, in a 53-gram gland. There were also scattered foci of Gleason pattern 5, but no perineural or lymphovascular invasion. His CT scan of the abdomen and pelvis, as well as whole body technicium-99m bone scan, were negative for metastatic disease.
The patient had a past history significant for cardiac valve replacements and coronary artery bypass grafting. He had also had a laminectomy and discectomy at the 3rd and 4th lumbar vertebrae. He was on no prescribed medications.
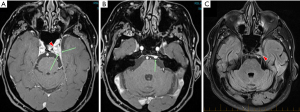
The patient was commenced on neoadjuvant ADT with the GnRH agonist, Triptorelin, in February 2018 and by April 2018 his PSA had dropped to 1.5. After developing a left abducens nerve palsy, he had a cranial MRI which was consistent with a meningioma of the posterior aspect of the left cavernous sinus (Figure 1). It was likely that the Triptorelin had stimulated growth of the meningioma and caused the nerve palsy. Triptorelin was ceased and the nerve palsy gradually resolved over a three-month period. The patient was subsequently commenced on a GnRH antagonist (Degarelix). The meningioma had a stable appearance on MRI over a 10-month period (not shown).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The sixth cranial (abducens) nerve arises from the brainstem at the junction of the pons and medulla oblongata, medial to the facial nerve. After traversing Dorello’s canal and at the tip of the petrous temporal bone, it enters the cavernous sinus lateral to the internal carotid artery, before passing through the superior orbital fissure to innervate the lateral rectus muscle (https://teachmeanatomy.info/). The lateral rectus muscle abducts the globe, hence a left lateral rectus palsy results in an inability to laterally deviate the eye to the left. The meningioma in our patient’s case was in the posterolateral part of the cavernous sinus and ADT therapy was associated with a clinical sixth cranial nerve palsy, presumably via compression of the abducens nerve by the enlarging meningioma. Once ADT therapy was discontinued, there was a gradual resolution of the cranial nerve palsy, suggesting decompression of the abducens nerve in the cavernous sinus, however the meningioma remained grossly the same size on serial MRI, over a period of 10 months.
Our patient did not have biopsy proof of the cavernous sinus lesion, although the lesion had all the MRI hallmarks of meningioma: a well-circumscribed lesion with homogeneous enhancement and a very limited differential diagnosis. It was very unlikely to be a schwannoma, on MRI criteria (Figure 1) and metastatic prostate cancer was likewise a very unlikely diagnosis.
Meningiomas have been well-described to be hormone-sensitive tumours; they are sensitive to fluctuations in female hormones. For example, they have a higher incidence in females, particularly during the reproductive years and there are multiple reports of exacerbation of meningioma symptoms during pregnancy (9-11). In some studies, the use of oral contraceptives and hormonal replacement therapy have both been linked to raised meningioma incidence (12,13), whereas in others, no such association was noted (14). In male to female transsexuals, meningioma induction has been described in association with anti-androgen therapies (15-17).
Meningiomas are also reported to harbour receptors for various hormones, including receptors for progesterone, oestrogen, prolactin, somatostatin, androgen and GnRH (3,7,8). Furthermore, LHRH (GnRH) has been shown, in vitro, to enhance the proliferation of human meningioma cells (18), whereas a progesterone-blocking agent, mifepristone, had the opposite effect, restricting meningioma proliferation and growth in vivo, in nude mice (19). Reflecting this, ADT has been reported in some prostate cancer patients to be associated with enhanced meningioma growth (4-6,20), to the extent that it has been recommended that ADT be judiciously used, or avoided, in patients with meningiomas (3,20). On the other hand, in a patient on ADT for benign prostatic hypertrophy, regression of a meningioma was observed after ADT cessation (21). However, GnRH treatment of prostate cancer does not universally appear to stimulate the growth of known meningiomas: Fallanca et al. (2009) (22) reported two such patients whose meningiomas did not clinically or radiologically progress over the course of at least a year of GnRH treatment. It was possible that their tumours did not express the appropriate hormone receptors. Careful meningioma monitoring during ADT therapy is however clearly indicated.
Cyproterone acetate is an antiandrogen and commonly used form of ADT; it is particularly useful in abrogating flares associated with initial GnRH use and in the treatment of locally advanced and metastatic prostate cancer (23). However, its use has not been associated with meningioma stimulation.
Choline-PET scanning was previously used routinely for the diagnosis, staging and determination of response in prostate cancer (Zhou et al., 2019) (24). These scans revealed many previously undiagnosed meningiomas (Fallanca et al., 2009 (22) and references therein), but choline-PET use has largely been superceded by PSMA-PET (23). PSMA-PET is rarely positive in meningioma (the usual test in meningioma being dotatate-PET, a scan for somatostatin receptors), but it can be positive in tumour types with neovascularisation (24,25), which would include the less common high-grade meningiomas. Our patient reacted adversely to a GnRH agonist, developing an abducens nerve palsy. He had high risk localised prostate cancer and so ADT was indicated; after the GnRH agonist was ceased, he was recently started on a GnRH antagonist (Degarelix), which in theory should provide prostate cancer growth restraint whilst not stimulating meningioma proliferation/growth (3). This may well be the most appropriate form of ADT for meningioma patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-57/rc
Peer Review File: Available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-57/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-57/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Harris WP, Mostaghel EA, Nelson PS, et al. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol 2009;6:76-85. [Crossref] [PubMed]
- Teo MY, Rathkopf DE, Kantoff P. Treatment of Advanced Prostate Cancer. Annu Rev Med 2019;70:479-99. [Crossref] [PubMed]
- Li Q, Coulson H, Klaassen Z, et al. Emerging association between androgen deprivation therapy and male meningioma: significant expression of luteinizing hormone-releasing hormone receptor in male meningioma. Prostate Cancer Prostatic Dis 2013;16:387-90. [Crossref] [PubMed]
- Anda T, Honda M, Ishihara T, et al. Progression of intracranial meningioma during luteinizing hormone-releasing hormone agonist treatment for prostate cancer: case report. Neurol Med Chir (Tokyo) 2014;54:327-30. [Crossref] [PubMed]
- Tsutsui T, Miyashita K, Sabit H, et al. Acute Progression of Recurrent Meningioma during Luteinizing Hormone-Releasing Hormone Agonist Treatment for Prostate Cancer. World Neurosurg 2016;91:670.e1-6. [Crossref] [PubMed]
- Fabbri VP, Asioli S, Palandri G. An unusual case of "sterile" abscess within low-grade meningioma during anti androgenic therapy and LH-releasing hormone agonist treatment for prostate cancer. Clin Neurol Neurosurg 2020;196:105993. [Crossref] [PubMed]
- Olson JJ, Beck DW, MacIndoe JW, et al. Androgen receptors in meningiomas. Cancer 1988;61:952-5. [Crossref] [PubMed]
- Hirota Y, Tachibana O, Uchiyama N, et al. Gonadotropin-releasing hormone (GnRH) and its receptor in human meningiomas. Clin Neurol Neurosurg 2009;111:127-33. [Crossref] [PubMed]
- Olson JJ, Beck DW, Schlechte J, et al. Hormonal manipulation of meningiomas in vitro. J Neurosurg 1986;65:99-107. [Crossref] [PubMed]
- Cea-Soriano L, Blenk T, Wallander MA, et al. Hormonal therapies and meningioma: is there a link? Cancer Epidemiol 2012;36:198-205. [Crossref] [PubMed]
- Wigertz A, Lönn S, Hall P, et al. Reproductive factors and risk of meningioma and glioma. Cancer Epidemiol Biomarkers Prev 2008;17:2663-70. [Crossref] [PubMed]
- Claus EB, Calvocoressi L, Bondy ML, et al. Exogenous hormone use, reproductive factors, and risk of intracranial meningioma in females. J Neurosurg 2013;118:649-56. [Crossref] [PubMed]
- Korhonen K, Auvinen A, Lyytinen H, et al. A nationwide cohort study on the incidence of meningioma in women using postmenopausal hormone therapy in Finland. Am J Epidemiol 2012;175:309-14. [Crossref] [PubMed]
- Custer B, Longstreth WT Jr, Phillips LE, et al. Hormonal exposures and the risk of intracranial meningioma in women: a population-based case-control study. BMC Cancer 2006;6:152. [Crossref] [PubMed]
- Gazzeri R, Galarza M, Gazzeri G. Growth of a meningioma in a transsexual patient after estrogen-progestin therapy. N Engl J Med 2007;357:2411-2. [Crossref] [PubMed]
- Deipolyi AR, Han SJ, Parsa AT. Development of a symptomatic intracranial meningioma in a male-to-female transsexual after initiation of hormone therapy. J Clin Neurosci 2010;17:1324-6. [Crossref] [PubMed]
- Bergoglio MT, Gómez-Balaguer M, Almonacid Folch E, et al. Symptomatic meningioma induced by cross-sex hormone treatment in a male-to-female transsexual. Endocrinol Nutr 2013;60:264-7. [Crossref] [PubMed]
- Lee KL, Terris MK. Luteinizing hormone-releasing hormone agonists and meningioma: a treatment dilemma. Urology 2003;62:351. [Crossref] [PubMed]
- Durmaz R, Deliorman S, Işiksoy S, et al. Luteinizing hormone releasing hormone increases proliferation of meningioma cells in vitro. Arch Physiol Biochem 1999;107:286-91. [PubMed]
- Olson JJ, Beck DW, Schlechte JA, et al. Effect of the antiprogesterone RU-38486 on meningioma implanted into nude mice. J Neurosurg 1987;66:584-7. [Crossref] [PubMed]
- Shimizu J, Matsumoto M, Yamazaki E, et al. Spontaneous regression of an asymptomatic meningioma associated with discontinuation of progesterone agonist administration. Neurol Med Chir (Tokyo) 2008;48:227-30. [Crossref] [PubMed]
- Fallanca F, Giovacchini G, Picchio M, et al. Incidental detection by 11Ccholine PET/CT of meningiomas in prostate cancer patients. Q J Nucl Med Mol Imaging 2009;53:417-21. [PubMed]
- Bastide C, Bruyère F, Karsenty G, et al. Hormonal treatment in prostate cancer. Prog Urol 2013;23:1246-57. [Crossref] [PubMed]
- Zhou J, Gou Z, Wu R, et al. Comparison of PSMA-PET/CT, choline-PET/CT, NaF-PET/CT, MRI, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: a systematic review and meta-analysis. Skeletal Radiol 2019;48:1915-24. [Crossref] [PubMed]
- Farag M, Bolton D, Lawrentschuk N. Prostate-specific membrane antigen for the surgical oncologist: interpreting expression beyond the prostate. ANZ J Surg 2020;90:715-8. [Crossref] [PubMed]
Cite this article as: McKay MJ, Pandey R, Zadeh H, Taubman K. Gonadotropin releasing hormone agonist-related meningioma progression causing abducens nerve palsy: an oncological case report. Precis Cancer Med 2022;5:40.

