Mechanisms of resistance after crizotinib or second-generation ALK therapy in advanced non-small cell lung cancer
Introduction
Anaplastic lymphoma kinase (ALK) gene rearrangements occur in ~5% of patients with advanced non-small cell lung cancer (NSCLC), mainly in lung adenocarcinomas. The first-generation ALK tyrosine kinase inhibitor (TKI), crizotinib, and, more recently the next generation ALK TKIs (second generation: ceritinib, ensartinib, alectinib, brigatinib and third generation: lorlatinib) have significantly enlarged the therapeutic arsenal in this population. Crizotinib was the first ALK TKI approved by both the FDA (2011) and EMA (2012), as standard treatment for lung cancers harboring ALK rearrangements. In the first-line setting, crizotinib improved the response rate (RR) and the progression free survival (PFS) compared with platinum-based chemotherapy in two randomised phase III clinical trials, the PROFILE 1014 (1,2) and the PROFILE 1029 (3), the former performed only in Asian patients. Similarly, the phase III ASCEND-4 trial (4) confirmed the efficacy of ceritinib compared with chemotherapy in the same subset of patients. However, the therapeutic strategy in first-line setting has shifted after significant improvement in PFS and meaningful higher intracranial activity with alectinib in the ALEX trial (5,6) and brigatinib in the ALTA-1L trial (7,8) were found when compared with crizotinib. Nowadays, both drugs are the new standards of care in the first-line setting. Alectinib was approved by the EMA on 12th October 2017 and by the FDA on 6th November 2017, whereas brigatinib in the same setting obtained only FDA approval on 26th May 2020. Recently, in the updated overall survival (OS) from the ALEX trial, for the first time an OS benefit of a next-generation ALK TKI was reported compared with crizotinib in the treatment naïve ALK-positive NSCLC patients, with a clinically meaningful 5-year OS rate of 63% achieved with alectinib versus 46% with crizotinib (6). Recently, advanced ALK-positive advanced NSCLC the phase III EXALT trial reported a significant longer PFS with ensartinib compared with crizotinib (25.8 vs. 12.7 months, hazard ratio, HR 0.51; 95% CI: 0.35–0.72), adding a new potential first-line treatment strategy. In the coming future, in this subset of patients, results of the ongoing phase III CROWN trial (lorlatinib vs. crizotinib) may shift the treatment paradigm once again. Efficacy and toxicity profile ratio, as well as intracranial activity with these agents may be relevant for making treatment decisions in the first-line setting.
Liquid biopsy identifies mechanisms of acquired resistance (AR) in ALK NSCLC
In ALK-positive NSCLC patients, despite initial response to ALK TKI, most patients eventually progress and acquired TKI resistance may still be driven by ALK-dependency. Of note, intra-tyrosine kinase ALK-mutations are the main mechanism of AR to ALK TKI, observed in up to one-third in crizotinib-refractory patients to 56% in patients progressing on second-generation ALK TKIs (9). Therefore, sequential ALK TKIs may be an optimal treatment option at the time of AR. In the PROFILE 1014 trial, the longest OS was observed in crizotinib-refractory patients who received subsequent ALK TKI at the time of progression (PD), reaching a 4-year OS of 80% compared with 25% for those without subsequent personalised treatment (2), supporting the relevance of sequencing strategies for improving patients’ outcome. Similarly, the retrospective French CLINALK study (10) and other cohorts (11) have reported a median OS of up to 7.5 years from metastatic disease diagnosis for those patients who received different ALK TKI in the therapeutic strategy after upfront crizotinib.
In the crizotinib-refractory population, all next generation ALK TKIs (brigatinib, ensartinib, ceritinib, lorlatinib) have reported activity (12-16). Indeed, in crizotinib-refractory tumors, the efficacy of next generation ALK TKIs is independent of the occurrence of acquired ALK-mutations (15,17-19), supporting blinded sequential strategies at crizotinib progression. However, the upfront administration of second generation ALK TKI based on the recent results from the ALEX (5,6) and ALTA-1L (7,8) studies have challenged the current sequential strategy with ALK TKI. In fact, blinded sequential strategies with a second-generation ALK TKI at the time of progression on previous second-generation ALK TKI would not be the most suitable strategy (20).
The optimal sequential treatment strategy at the time of progression on ALK TKI may be relevant, as each ALK TKI appears to be associated with a specific acquired ALK mutation profile. This is very relevant for the acquired G1202R ALK-mutation, which confers high-level of resistance to first- and second-generation ALK TKIs. Although it is an uncommon event in post-crizotinib (~2%) tumor-samples (9), the G1202R mutation occurs in up to 50% of tumors at progression on second-generation ALK TKI (15). Indeed, it has different incidences according to the previous second-generation ALK TKI (21% post-ceritinib, 29% post-alectinib, and 43% post-brigatinib) (9) and at the moment can only be effectively overcome by lorlatinib (15). Therefore, as not all second-generation ALK TKI homogeneously bypass all acquired ALK-mutations (9), tumor genotyping at progression may help to implement tailored approaches. However, almost one-third of advanced NSCLC patients do not have adequate tumor tissue for genomic profiling (21), and liquid biopsy (analysis of circulating tumor DNA, ctDNA) is a reliable and alternative tool for genomic profiling in NSCLC (22). In some ALK cohorts, 76% of plasma samples contained sufficient tumor-derived DNA for molecular analysis, compared with 65% of biopsy specimens, confirming that both are reliable approaches (23). In ALK-positive NSCLC, similarly to other oncogenic addicted NSCLC, liquid biopsies are informative for knowing the broad genomic profile at baseline or at the time of progression (15,23-28). They are also useful to monitor the dynamic evolution of resistance mechanisms upon ALK TKIs based on longitudinal ctDNA analyses (23) allowing personalised treatment approaches according to the ALK mutation resistant portrait (23,29). However, liquid biopsy sensitivity to detect genomic alterations is closely related to clinical factors such as stage and metastatic tumor burden, suggesting limited shed of tumor DNA in cases missed by plasma genotyping (30).
Currently, most reports for detection of ALK mutation in ctDNA have been made with next generation sequencing (NGS). Moreover, the wide range of mutations that have to be covered and the number of upcoming new drugs suggest that NGS will be the optimal method for determination of ALK mutations from ctDNA (31). Recently, it has been reported that NGS plasma genotyping for any ALK mutations using as reference ALK mutation status in de novo tumor tissue biopsy has a sensitivity and a specificity of 61% and 82%, respectively, with an overall agreement of 73% (15). However, in other series the agreement rate has reached 100% by amplicon-based NGS (25) or hybrid-capture NGS (23). Indeed, contrary to single-site biopsy specimens, liquid biopsy may capture the spatial heterogeneity of ALK mutations that may exist in subclones of tumors (23-25), and may even provide an advantage above tissue analysis.
The detection rate of ALK mutations in ctDNA after ALK TKI failure ranges from 11% to 66% depending on the potency of previous ALK TKI (15,23,25-27), with higher incidence of ALK mutations after next-generation ALK TKIs (25,27). Indeed, the detection of ≥2 ALK mutations is significantly more common in patients relapsing on lorlatinib compared with second-generation ALK TKIs (48% vs. 23%, P=0.017), and ctDNA analysis rather than tissue analysis has higher capability to identify these compound mutations (27). Finally, the detection of ctDNA at the time of ALK TKI-failure may correlate with the prognosis of the disease. In an exploratory analysis, the absence of mutations in ctDNA was associated with improved outcomes compared with those patients with at least one ALK mutation (median OS: 105 vs. 58.5 months, P=0.001); and this effect could be related to a lower tumor burden or a less heterogeneous tumor (25).
Others than ALK mutations have been reported in liquid biopsy as mechanisms of AR, such as MET amplification. When tissue was used as the reference, plasma genotyping demonstrated 100% sensitivity, 95% specificity, and 80% positive predictive value for detecting MET amplification (28).
Intracranial progression on ALK TKI in ALK-positive patients is a real challenge (32). For those patients with isolated intracranial progression the detection rate of genomic alterations by ctDNA analyses is lower than among those patients with other metastatic sites of progression (33). Of note, ctDNA ALK mutations are detected in only 10% of cases (N=3/29) with isolated central nervous system (CNS) relapse compared with ~75% of ALK mutations detected by ctDNA analysis in patients with liver or bone metastases (25). In contrast, another cohort reported an ALK mutation and/or ALK fusion in plasma in 89% of patients with confined intracranial or intrathoracic relapse (N=17/19) (27). Subgroup analysis according to intracranial or intrathoracic relapse was not provided. Of note, lumbar puncture with genotyping of cerebrospinal fluid is also becoming an option in those patients with isolated intracranial progression (34,35). When possible, tumor re-biopsy for genotyping is recommended in cases of a negative plasma profile as well as to rule out histologic transformation as mechanism of resistance (36-38).
Liquid biopsy in post-crizotinib setting
In the registration multicohort phase II study of lorlatinib, baseline plasma and tumor samples were collected from 198 ALK-positive NSCLC patients. Plasma ctDNA was analysed by 73-genes NGS assay (Guardant 360) and tumor tissue was profiled using a central, customized NGS assay on the Ion Torrent PGM platform at Molecular MD (Portland, OR). Fifty-nine patients had received prior crizotinib +/‒ chemotherapy (27 patients only prior crizotinib; and 32 prior crizotinib and chemotherapy), whereas 139 patients had received one or more second-generation ALK TKIs, often with crizotinib preceding the second-generation ALK inhibitor. In the whole cohort, among 189 ALK-positive patients with baseline plasma genotyping, 24% had one or more ALK mutation detectable in ctDNA, and 21% of patients had no detectable ctDNA (15). In tissue, ALK-mutations were detected in 24% of 198 tumor-samples (archival and de novo biopsies), however, in de novo tumor samples, ALK mutation incidence reached 47%. The ~25% of ctDNA ALK mutation detection rate in this study at TKI failure (15) is similar to data reported in other cohorts (25), however, lower than reported in tissue, which could be justified by lack of tumor shedding into the blood.
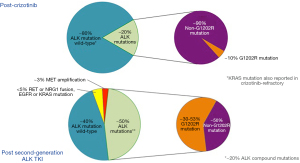
In the whole cohort from lorlatinib study, based on plasma genotyping, the most common ALK mutations were G1202R (42%), L1196M (24%), F1174X (24%), G1269A (18%), and I1171X (11%). However, according to previous ALK TKI, in the post-crizotinib setting [11 out of 59 (19%) had detectable ALK mutations and 44 patients (77%) did not], the most common ALK mutations were G1269A, F1174X, and L1196M, whereas G1202R was detected in a minority of cases. In the post-crizotinib setting, the efficacy of lorlatinib occurred regardless of the presence or absence of detectable ctDNA ALK mutations (Figure 1). Lorlatinib reported a RR of 73% among mutation-positive patients and 75% among mutation-negative patients, with no differences in median PFS between these subgroups [NR vs. 12.5 months, respectively; HR 1.38, 95% CI: 0.48 to 3.98] (15). In two other cohorts of crizotinib-refractory patients, ALK mutations assessed by ctDNA analyses were identified in up to 29% of cases (24,26), being the most common the L1196M (26), the G1269A and the S1206F (24). Contrary, in another cohort, the amplicon-based NGS assay only reported 11% of ALK mutations in post-crizotinib patients (including 1 case with G1202R). Globally, the incidence of ALK mutations in crizotinib-resistant tumors is low (~15–20%). Differences in the incidence of ALK mutations in the post-crizotinib setting may reflect the different threshold sensitivity for detecting ALK mutations of the broad techniques used for genomic profiling. These data reinforce that crizotinib-resistant tumors, including those without a detectable ALK mutation, are still driven by ALK and remain responsive to more potent ALK inhibitors. In this scenario, all next generation ALK TKIs have reported activity (12-16), putting into question the utility of genomic profiling in crizotinib-refractory tumors. Although no head to head comparison between second-generation ALK TKI is still available, some differences may exist (39). The ongoing ALTA-3 trial (NCT03596866) assesses the efficacy of brigatinib versus alectinib in crizotinib-refractory ALK-positive NSCLC patients and may help to elucidate the best sequential treatment strategy in these patients. Although the incidence of acquired G1202R in the post-crizotinib setting is low, genomic profiling in this setting would be relevant as it could suggest sequential treatment with lorlatinib if this mutation occurs.
Other than ALK mutations have also been reported as mechanisms of AR to crizotinib in ctDNA analysis. According to digital drop PCR (ddPCR) cfDNA assay, KRAS mutation occurred in 10 out of 20 crizotinib-refractory patients (7 p.G12D, 2 p.G12V, and 1 p.G12C mutations, respectively). In 3 patients KRAS mutations were associated with ALK mutations. ctDNA was monitored during the treatment with second generation ALK-inhibitors and the amount of both ALK or KRAS mutations decreased along with tumor regression (40). MET amplification only occurred as mechanism of AR in crizotinib-refractory in 9% of tumors, either in tissue or liquid biopsy genotyping (28).
Liquid biopsy in post next-generation ALK TKI setting
Nowadays, sequential treatment strategies after upfront second-generation ALK TKI are challenging, as not all ALK TKIs available have activity in this setting and the high risk of acquiring the G1202R mutation may limit the potential sequential (9). Blinded sequential ALK TKI strategy with a second-generation ALK TKI upon progression on previous second generation ALK TKI have reported limited outcomes (RR ~30% and median PFS of ~4 months) (20). The ongoing phase II ALTA2 trial (NCT03535740) assessing brigatinib efficacy in alectinib- or ceritinib-refractory ALK-positive NSCLC patients may further help to define the role of a blinded-sequential strategy with brigatinib after second generation ALK TKI failure.
Evidence of mechanisms of AR after second-generation ALK TKI, either ALK-dependent resistance or ALK-independent resistance occurring in up to 50% of cases (41), come from tissue or liquid biopsies after progression on these agents in second-line setting (Figure 1). Whether the resistance pattern may differ when second-generation ALK TKI are administered in the first-line setting remains unknown and it is challenging, as these drugs are the new standard of care in treatment-naïve ALK-positive NSCLC patients.
ALK mutations are the key driver of AR after second-generation ALK TKI, with an incidence in liquid biopsy ranging from 27% to 66% (23-25,27), being the most common acquired ALK mutation the G1202R detected in ctDNA in 30% to 53% of cases (15,23,27). Likewise, up to 25% of plasma specimens contain ≥2 ALK mutations regardless of previous number of second-generation ALK TKI (25% vs. 19% in one versus multiple second-generation ALK TKI, respectively, P<0.743) (27). The proportion of patients relapsing on second-generation ALK TKI due to secondary ALK mutations is similar based on tissue or plasma genotyping, when both biopsies are collected in the same time-period (15,27). In alectinib-resistant tumors, 67% and 63% of patients had an ALK-mutation in plasma or tissue genotyping, respectively. ALK-mutation in plasma versus tissue genotype in alectinib-refractory tumors included G1202R (37% vs. 24%), I1171X (26% vs. 24%), L1196M (22% vs. 2%) and V1180L (11% vs. 10%). However, plasma genotyping was significantly more likely than tissue genotyping to identify a subset of alectinib-resistant cancers harboring ≥2 ALK mutations (24% in plasma vs. 2% in tissue, P<0.004) (27). This is of relevance as compound ALK mutations are associated with shorter PFS and OS (25) and these compound mutations may affect the efficacy of lorlatinib. Compound ALK mutations detected in tissue had lower RR with lorlatinib compared with patients with only one ALK mutation (56% vs. 75%, respectively), and shorter median duration of response (6.1 vs. 24.4 months, respectively). This correlation has not been assessed in ctDNA as the numbers were small based on plasma genotyping (15). Indeed some compound ALK mutations hamper sequential lorlatinib efficacy (42). Therefore, the identification of these mutations has relevant clinical implications for making treatment decisions, as some compound ALK secondary mutations such us the L1198-containing compound mutations seem to be resistant to next-generations ALK-TKI, but sensitive to crizotinib (42,43).
The occurrence of ALK mutations in ctDNA among patients who have failed to one or more second-generation ALK TKI may be prognostic. Although lorlatinib has reported clinically meaningful efficacy in this subset (RR 40%, PFS 6.9 months), on the basis of plasma genotyping, ALK mutation-positive patients in ctDNA reported higher RR with lorlatinib (62% vs. 32%) and longer PFS (7.3 vs. 5.5 months) compared with those mutation-negative (15). These data suggest that, ALK mutations may identify tumors with continued ALK dependency after second-generation ALK TKI, and the absence of an ALK mutation suggests the potential development of ALK-independent mechanisms of resistance, making them less likely to respond to ALK inhibition. However, ALK-mutation negative patients are not excluded for receiving treatment with lorlatinib. Likewise, the occurrence of co-mutations detected in ctDNA may be relevant, such as the TP53 mutation in up to 50% of cases, which correlates with shorter PFS on ALK TKI (9,24-26). Therefore, liquid biopsy may be informative about the occurrence of co-mutations that may negatively impact in patients’ outcome. In the end, liquid biopsy results could also be used to select patients that would benefit more from chemotherapy compared with a next line of ALK TKI.
Considering the crucial prognostic and predictive value of secondary ALK resistance mutations subtype for the selection of the optimal sequential ALK-TKI, serial ctDNA analysis may provide real-time information on the disease molecular evolution upon ALK-TKI therapy. This information may guide clinicians in their sequencing approaches instead of blinded treatment decisions. However, the impact in patients’ outcome with tailored approach after progression on second-generation ALK TKIs remains unknown. Two prospective ongoing studies, the NCI-NRG ALK MASTER protocol (NCT03737994) and the EORTC-ALKALINE protocol (NCT04127110) are currently exploring the application of liquid biopsy in this setting.
ALK independent mechanisms of AR are also relevant as some can be overcome with personalised strategies. These include NRG1 and RET gene fusion, and EGFR or KRAS mutations. Also, it has been identified mutations in IDH1, NOTCH and NF1 (26). Gene fusions are relevant as tarloxotinib, and selpercatinib or pralsetinib have reported activity in NRG1- and RET-fusion tumors, respectively (44,45). In other oncogenic addicted tumors, such as EGFR-mutant NSCLC and RET-mediated resistance, the combination of osimertinib and pralsetinib was well tolerated and led to rapid radiographic response, supporting that combination of EGFR and RET TKI may be a good strategy to overcome this mechanisms of AR (46). This therapeutic strategy in ALK tumors remains unknown, but detection of RET-fusions by ctDNA is a valid screening strategy (47).
In tissue biopsy, MET amplification was detected in 15% of tumor biopsies from patients relapsing on next-generation ALK TKI. Of note, tumors from patients previously treated with crizotinib followed by next-generation TKIs were significantly less likely to harbor MET amplification than those from patients treated only with next generation ALK TKI (9% vs. 33%, P<0.019), as crizotinib has anti-MET activity (48-50) reducing the emergence of MET amplification clones in these patients. In liquid biopsy, MET amplification frequency was 7%. Although in tissue biopsy MET amplification was mutually exclusive with ALK resistance mutations, in ctDNA analysis, half of specimens with focal MET amplification harbored both an ALK mutation and MET amplification in plasma. This could suggest that the tumor becomes more heterogeneous with different resistance mechanisms occurring in different tumor sites. Similar to EGFR mutant tumors, there exists an association between ALK TKI potency and the likelihood of developing ALK-independent resistance mechanisms such as MET amplification, being higher after lorlatinib than after second-generation ALK TKI either in tissue (22% vs. 12%) or in liquid biopsy (17% vs. 3%) (Figure 1) (28).
Previous data suggested that the up-front administration of third generation ALK-TKI could prevent the onset of on-target resistance mutations, potentially improving patients’ clinical outcomes (42), but upfront treatment with next-generation ALK TKI may lead to MET-driven resistance in one-third of cases (28). This is a real challenge as second-generation ALK TKIs are the new standard of care in first-line setting, and MET-amplification may become a mechanism of AR in up to one third of cases. Defining the optimal threshold of MET amplification for predicting sensitivity to MET inhibitors is a current challenge, as well as defining the role of dual ALK and MET inhibitors. Whether this should be either with anti-MET TKI, antidrug conjugated or antibodies with MET inhibition remain unknown.
Conclusions
Although acquired ALK mutation remains the major mechanism of AR after next generation ALK TKI, with the G1202R as most common ALK mutation in half of cases, MET amplification occurs in up to one third of tumors not previously treated with crizotinib. RET-fusion is another druggable mechanism of AR in ALK-positive NSCLC. All of these mechanisms can be identified in a liquid biopsy, enlarging the proportion of patients with a genomic portrait at the time of progression on TKI that may get benefit of a sequential tailored treatment. New treatment strategies, mainly dual combinations anti-ALK and anti-MET as well as anti-ALK and anti-fusions (RET, NRG1) are future challenges in this subset of lung cancers.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Jesús Corral, Laura Mezquita and Ernest Nadal) for the series “ALK and ROS-1 NSCLC Patients Treatment Approach Based on Genomic Profile by Liquid Biopsy” published in Precision Cancer Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pcm.amegroups.com/article/view/10.21037/pcm-20-33/coif). The series “ALK and ROS-1 NSCLC Patients Treatment Approach Based on Genomic Profile by Liquid Biopsy” was commissioned by the editorial office without any funding or sponsorship. LELH. serves as an unpaid editorial board member of Precision Cancer Medicine from February 2021 to January 2023. LELH reports other from boehringer ingelheim, other from BMS, other from Roche Genentech, other from BMS, grants from Roche Genentech, grants from Boehringer Ingelheim, other from AstraZeneca, personal fees from Quadia, grants from Astra Zeneca, other from Eli Lilly, other from Roche Genentech, other from Pfizer, other from MSD, other from Takeda, non-financial support from AstraZeneca, non-financial support from Novartis, non-financial support from BMS, non-financial support from MSD/Merck, non-financial support from GSK, non-financial support from Takeda, non-financial support from Blueprint Medicines, non-financial support from Roche Genentech, outside the submitted work. JR reports other from MSD, other from BOEHRINGER, other from PFIZER, personal fees and other from OSE IMMUNOTHERAPEUTICS, other from BMS, other from ASTRAZENECA, other from ROCHE, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Solomon BJ, Kim DW, Wu YL, et al. Final Overall Survival Analysis From a Study Comparing First-Line Crizotinib Versus Chemotherapy in ALK-Mutation-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:2251-8. [Crossref] [PubMed]
- Wu YL, Lu S, Lu Y, et al. Results of PROFILE 1029, a Phase III Comparison of First-Line Crizotinib versus Chemotherapy in East Asian Patients with ALK-Positive Advanced Non-Small Cell Lung Cancer. J Thorac Oncol 2018;13:1539-48. [Crossref] [PubMed]
- Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389:917-29. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Mok T, Camidge DR, Gadgeel SM, et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol 2020;31:1056-64. [Crossref] [PubMed]
- Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus Crizotinib in ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2027-39. [Crossref] [PubMed]
- Camidge R, Kim HR, Ahn MJ, et al. Brigatinib vs crizotinib in patients with ALK inhibitor-naive advanced ALK+ NSCLC: Updated results from the phase III ALTA-1L trial. Ann Oncol 2019;30:ix195-6. [Crossref]
- Gainor JF, Dardaei L, Yoda S, et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov 2016;6:1118-33. [Crossref] [PubMed]
- Duruisseaux M, Besse B, Cadranel J, et al. Overall survival with crizotinib and next-generation ALK inhibitors in ALK-positive non-small-cell lung cancer (IFCT-1302 CLINALK): a French nationwide cohort retrospective study. Oncotarget 2017;8:21903-17. [Crossref] [PubMed]
- Pacheco JM, Gao D, Smith D, et al. Natural History and Factors Associated with Overall Survival in Stage IV ALK-Rearranged Non-Small Cell Lung Cancer. J Thorac Oncol 2019;14:691-700. [Crossref] [PubMed]
- Shaw AT, Kim TM, Crinò L, et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2017;18:874-86. [Crossref] [PubMed]
- Huber RM, Hansen KH, Paz-Ares Rodríguez L, et al. Brigatinib in Crizotinib-Refractory ALK+ NSCLC: 2-Year Follow-up on Systemic and Intracranial Outcomes in the Phase 2 ALTA Trial. J Thorac Oncol 2020;15:404-15. [Crossref] [PubMed]
- Novello S, Mazières J, Oh IJ, et al. Alectinib versus chemotherapy in crizotinib-pretreated anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer: results from the phase III ALUR study. Ann Oncol 2018;29:1409-16. [Crossref] [PubMed]
- Shaw AT, Solomon BJ, Besse B, et al. ALK Resistance Mutations and Efficacy of Lorlatinib in Advanced Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2019;37:1370-9. [Crossref] [PubMed]
- Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol 2018;19:1654-67. [Crossref] [PubMed]
- Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014;370:1189-97. [Crossref] [PubMed]
- Gettinger SN, Zhang S, Hodgson JG, et al. Activity of brigatinib (BRG) in crizotinib (CRZ) resistant patients (pts) according to ALK mutation status. J Clin Oncol 2016;34:9060. [Crossref]
- Wolf J, Helland Å, Oh I, et al. Phase 3 ALUR Study of Alectinib in Pretreated ALK+ NSCLC: Final Efficacy, Safety and Targeted Genomic Sequencing Analyses. J Thorac Oncol 2019;14:S210. [Crossref]
- Remon J, Tabbò F, Jimenez B, et al. Sequential blinded treatment decisions in ALK-positive non-small cell lung cancers in the era of precision medicine. Clin Transl Oncol 2020;22:1425-9. [Crossref] [PubMed]
- Chouaid C, Dujon C, Do P, et al. Feasibility and clinical impact of re-biopsy in advanced non small-cell lung cancer: a prospective multicenter study in a real-world setting (GFPC study 12-01). Lung Cancer 2014;86:170-3. [Crossref] [PubMed]
- Gadgeel SM, Mok TSK, Peters S, et al. Phase II/III blood first assay screening trial (BFAST) in patients (pts) with treatment-naïve NSCLC: Initial results from the ALK+ cohort. Ann Oncol 2019;30:v918. [Crossref]
- Dagogo-Jack I, Brannon AR, Ferris LA, et al. Tracking the Evolution of Resistance to ALK Tyrosine Kinase Inhibitors through Longitudinal Analysis of Circulating Tumor DNA. JCO Precis Oncol 2018; [Crossref] [PubMed]
- Horn L, Whisenant JG, Wakelee H, et al. Monitoring Therapeutic Response and Resistance: Analysis of Circulating Tumor DNA in Patients With ALK+ Lung Cancer. J Thorac Oncol 2019;14:1901-11. [Crossref] [PubMed]
- Mezquita L, Swalduz A, Jovelet C, et al. Clinical Relevance of an Amplicon-Based Liquid Biopsy for Detecting ALK and ROS1 Fusion and Resistance Mutations in Patients With Non-Small-Cell Lung Cancer. JCO Precis Oncol 2020; [Crossref] [PubMed]
- McCoach CE, Blakely CM, Banks KC, et al. Clinical Utility of Cell-Free DNA for the Detection of ALK Fusions and Genomic Mechanisms of ALK Inhibitor Resistance in Non-Small Cell Lung Cancer. Clin Cancer Res 2018;24:2758-70. [Crossref] [PubMed]
- Dagogo-Jack I, Rooney M, Lin JJ, et al. Treatment with Next-Generation ALK Inhibitors Fuels Plasma ALK Mutation Diversity. Clin Cancer Res 2019;25:6662-70. [Crossref] [PubMed]
- Dagogo-Jack I, Yoda S, Lennerz JK, et al. MET Alterations Are a Recurring and Actionable Resistance Mechanism in ALK-Positive Lung Cancer. Clin Cancer Res 2020;26:2535-45. [Crossref] [PubMed]
- Ding M, Deng L, Yu R, et al. Case Report: Temporal Heterogeneity of ALK Activating Mutations in Sequential ALK TKI-Treated Non-Small-Cell Lung Cancer Revealed Using NGS-Based Liquid Biopsy. Clin Lung Cancer 2019;20:e229-32. [Crossref] [PubMed]
- Paweletz CP, Lau CJ, Oxnard GR. Does Testing Error Underlie Liquid Biopsy Discordance? JCO Precis Oncol 2019; [Crossref] [PubMed]
- Rolfo C, Mack PC, Scagliotti GV, et al. Liquid Biopsy for Advanced Non-Small Cell Lung Cancer (NSCLC): A Statement Paper from the IASLC. J Thorac Oncol 2018;13:1248-68. [Crossref] [PubMed]
- Johung KL, Yeh N, Desai NB, et al. Extended Survival and Prognostic Factors for Patients With ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastasis. J Clin Oncol 2016;34:123-9. [Crossref] [PubMed]
- Aldea M, Hendriks L, Mezquita L, et al. Circulating Tumor DNA Analysis for Patients with Oncogene-Addicted NSCLC With Isolated Central Nervous System Progression. J Thorac Oncol 2020;15:383-91. [Crossref] [PubMed]
- Boire A, Brandsma D, Brastianos PK, et al. Liquid biopsy in central nervous system metastases: a RANO review and proposals for clinical applications. Neuro Oncol 2019;21:571-84. [Crossref] [PubMed]
- Seoane J, De Mattos-Arruda L, Le Rhun E, et al. Cerebrospinal fluid cell-free tumour DNA as a liquid biopsy for primary brain tumours and central nervous system metastases. Ann Oncol 2019;30:211-8. [Crossref] [PubMed]
- Gong J, Gregg JP, Ma W, et al. Squamous Cell Transformation of Primary Lung Adenocarcinoma in a Patient With EML4-ALK Fusion Variant 5 Refractory to ALK Inhibitors. J Natl Compr Canc Netw 2019;17:297-301. [Crossref] [PubMed]
- Park S, Han J, Sun JM. Histologic transformation of ALK-rearranged adenocarcinoma to squamous cell carcinoma after treatment with ALK inhibitor. Lung Cancer 2019;127:66-8. [Crossref] [PubMed]
- Levacq D, D'Haene N, de Wind R, et al. Histological transformation of ALK rearranged adenocarcinoma into small cell lung cancer: A new mechanism of resistance to ALK inhibitors. Lung Cancer 2016;102:38-41. [Crossref] [PubMed]
- Reckamp K, Lin HM, Huang J, et al. Comparative efficacy of brigatinib versus ceritinib and alectinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small cell lung cancer. Curr Med Res Opin 2019;35:569-76. [Crossref] [PubMed]
- Bordi P, Tiseo M, Rofi E, et al. Detection of ALK and KRAS Mutations in Circulating Tumor DNA of Patients With Advanced ALK-Positive NSCLC With Disease Progression During Crizotinib Treatment. Clin Lung Cancer 2017;18:692-7. [Crossref] [PubMed]
- Lin JJ, Riely GJ, Shaw AT. Targeting ALK: Precision Medicine Takes on Drug Resistance. Cancer Discov 2017;7:137-55. [Crossref] [PubMed]
- Yoda S, Lin JJ, Lawrence MS, et al. Sequential ALK Inhibitors Can Select for Lorlatinib-Resistant Compound ALK Mutations in ALK-Positive Lung Cancer. Cancer Discov 2018;8:714-29. [Crossref] [PubMed]
- Shaw AT, Friboulet L, Leshchiner I, et al. Resensitization to Crizotinib by the Lorlatinib ALK Resistance Mutation L1198F. N Engl J Med 2016;374:54-61. [Crossref] [PubMed]
- Tirunagaru VG, Estrada-Bernal A, Yu H, et al. Abstract: Tarloxotinib exhibits potent activity in NRG1 fusion and rearranged cancers. AACR Annual Meeting 2019; March 29-April 3, 2019; Atlanta, GA.
- Subbiah V, Cote GJ. Advances in Targeting RET-Dependent Cancers. Cancer Discov 2020;10:498-505. [Crossref] [PubMed]
- Piotrowska Z, Isozaki H, Lennerz JK, et al. Landscape of Acquired Resistance to Osimertinib in EGFR-Mutant NSCLC and Clinical Validation of Combined EGFR and RET Inhibition with Osimertinib and BLU-667 for Acquired RET Fusion. Cancer Discov 2018;8:1529-39. [Crossref] [PubMed]
- Rich TA, Reckamp KL, Chae YK, et al. Analysis of Cell-Free DNA from 32,989 Advanced Cancers Reveals Novel Co-occurring Activating RET Alterations and Oncogenic Signaling Pathway Aberrations. Clin Cancer Res 2019;25:5832-42. [Crossref] [PubMed]
- Camidge D, Otterson G, Clark JW, et al. Crizotinib in patients (pts) with MET-amplified non-small cell lung cancer (NSCLC): Updated safety and efficacy findings from a phase 1 trial. J Clin Oncol 2018;36:9062. [Crossref]
- Landi L, Chiari R, Tiseo M, et al. Crizotinib in MET-Deregulated or ROS1-Rearranged Pretreated Non-Small Cell Lung Cancer (METROS): A Phase II, Prospective, Multicenter, Two-Arms Trial. Clin Cancer Res 2019;25:7312-9. [Crossref] [PubMed]
- Moro-Sibilot D, Cozic N, Pérol M, et al. Crizotinib in c-MET- or ROS1-positive NSCLC: results of the AcSé phase II trial. Ann Oncol 2019;30:1985-91. [Crossref] [PubMed]
Cite this article as: Remon J, Esteller L, Hendriks LEL. Mechanisms of resistance after crizotinib or second-generation ALK therapy in advanced non-small cell lung cancer. Precis Cancer Med 2022;5:4.