
Prolonged immune checkpoint inhibitor response of a patient with a RET rearranged non-small cell lung cancer and high tumour mutational burden: case report and review of the literature
Introduction
Immune checkpoint inhibitors (ICI) have become the new standard of care (SoC) in the treatment of metastatic non-small cell lung cancer (NSCLC) patients. First, in second line and beyond, the programmed death-1 (anti-PD-1) antibodies nivolumab (registration regardless of PD-L1 status) and pembrolizumab (for PD-L1 positive patients) became available, followed by the anti-PD-L1 antibody atezolizumab (registration regardless of PD-L1) (1). Recently, ICI have also become SoC in the first line treatment of patients having a metastatic NSCLC without an activating epidermal growth factor receptor (EGFR) mutation or anaplastic lymphoma kinase (ALK) rearrangement. Pembrolizumab monotherapy is registered as first-line therapy for patients with a PD-L1 tumour proportion score (TPS) ≥50% (Europe) or ≥1% (USA) (1-5). Combinations of platinum-based chemotherapy and anti-PD-(L)1 inhibitors (pembrolizumab or atezolizumab, the latter combined with bevacizumab) have become SoC treatment regardless of PD-L1 status.
Data is limited regarding ICI efficacy in those with targetable oncogenic-driven advanced NSCLC. Markers generally associated with ICI benefit are PD-L1 expression and tumour mutational burden (TMB). However, PD-L1 is often constitutively expressed in tumours with an oncogenic driver, and correlation with response is often poor (5,6). In general, TMB is low in non-smoking related oncogenic drivers, such as EGFR and ALK (6,7). Most ICI efficacy data come from patients with EGFR mutated NSCLC and to a lesser extent from patients with mutations in more rare driver genes (e.g., ALK, ROS proto-oncogene 1 (ROS1), rearranged during transfection (RET) fusion, ERBB2 receptor tyrosine kinase 2 [ERBB2, also known as HER2), BRAF serine/threonine kinase proto-oncogene (BRAF) and MET proto-oncogene (MET)]. For these rare driver genes, mainly retrospective data exist (6). In general, in NSCLC of non-smokers harbouring oncogenic driver mutations, responses are low and long-term outcome is poor (8). Based on a subgroup analysis of the IMpower 150 trial (n=80 tumours with sensitizing EGFR mutations, n=34 with ALK rearrangements) it is stated in the European Society for Medical Oncology (ESMO) metastatic NSCLC guideline that EGFR mutated and ALK rearranged tumour patients can be treated with a combination of chemotherapy and ICI (platinum doublet/bevacizumab/atezolizumab) after exhaustion of all available TKI’s (1,9). BRAF V600 mutated patients can be treated with ICI (with or without chemotherapy) upon progression on dabrafenib/trametinib. For the rarer oncogenic drivers, no recommendation regarding ICI has been made (1).
The preferred first line treatment for a patient with NSCLC containing a targetable oncogenic driver mutation (EGFR, ALK, ROS1, BRAF) is a (registered) tyrosine kinase inhibitor (TKI). For other driver gene mutations, TKIs are only available in a clinical trial and recruitment into open trials is encouraged (1).
An example of a rare oncogenic driver, for which no TKIs have been approved yet, is the RET gene rearrangement, although several TKIs are under investigation. Choosing the right treatment may be challenging in these patients, especially when no RET-TKI is available within a clinical trial.
We present a case of a patient with a stage IV NSCLC with a low PD-L1 expression, high TMB and a RET gene rearrangement, as identified by fluorescence in situ hybridisation (FISH) analysis, who progressed after first line chemotherapy. We will describe the challenges in choosing the best second line treatment for this patient. After description of the case, we will summarize the current evidence for ICI in RET gene rearranged positive NSCLC, as well as the literature for RET-TKIs.
We present the following article in accordance with the CARE reporting checklist available at http://dx.doi.org/10.21037/pcm-2020-potb-03.
Case presentation
A 65-year old man, diagnosed with a cT4N3M1b stage IVB (TNM7) poorly differentiated NSCLC (differentiation between adenocarcinoma and squamous cell carcinoma initially not possible), was referred in March 2017 to our clinic for a second opinion regarding treatment options because of his recently diagnosed NSCLC. He had no relevant medical history, except for a history of smoking (100 pack years). At initial diagnosis, he had several bone and liver metastases. At that time, despite two attempts to obtain enough tissue, molecular analysis was not possible due to poor DNA quality in the obtained tissue samples. Liquid biopsy for molecular analysis was not available in the clinic at that time. After discussion with the patient, no third attempt to obtain tissue for molecular analysis was performed. First line clinical trials were not enrolling, and first line treatment with gemcitabin/cisplatin was started, with stable disease after four cycles (August 2017). In September 2017 he presented with disease progression, and gave consent to obtain a new biopsy for molecular analysis, as well as a biopsy for the CPCT-02 trial (NCT01855477). This is a trial in which fresh biopsies and blood samples are collected from patients with metastatic cancer, which are then analysed by whole genome sequencing (WGS) to obtain a mutational profile. The histological biopsy revealed a NSCLC, subtype adenocarcinoma, TTF-1 positive. The in-hospital molecular analysis showed no EGFR, KRAS, BRAF and MET-exon 14 skipping mutations (tested with PCR and pyrosequencing). PD-L1 [immunohistochemistry (IHC), 22C3 clone] expression in tumour cells was less than 1%. FISH showed no ROS1 nor ALK rearrangement and no MET amplification was detected. FISH analysis for the detection of RET gene rearrangements was performed using the Vysis 10q11 RET Break-Apart FISH Probe (Abbott Laboratories, Chicago, Illinois, USA), which revealed loss of the green probe signal, located at the 5’ centromere side of the common RET translocation breakpoint, in ~58% of the tumour cells, while the red probe signal at the 3’ telomere side of the gene (near the tyrosine kinase domain) was maintained. The latter indicates the presence of a RET gene rearrangement. At that time, no clinical trials involving RET-TKI’s were available in which the patient could be included. In October 2017, it was therefore decided to start ICI (nivolumab) as a second line therapy. During treatment, WGS results from the CPCT-02 trial became available, and it turned out that the patient’s tumour material had a high TMB, as indicated by the presence of 527 non-synonymous somatic missense mutations per whole genome DNA (cut-off for high TMB: >140) (10). Among the detected genomic variants, a single frame shift mutation located in the RB1 gene [c.1768delT, p.(Cys590fs)] was identified, and considered pathogenic based on the mutation type, as well as the detected variant allele frequency in relation to the tumour cell percentage (~40%). The mutation had a variant allele frequency of 36%, most likely suggestive for loss of heterozygosity (LOH) of the other RB1 allele. After 7 cycles of nivolumab the CT-scan showed a nearly complete remission. After 2 years of treatment, the nivolumab was discontinued because of a persistent grade 2 arthritis, despite treatment with methotrexate and low dose corticosteroids. Nine months after discontinuation of nivolumab, the patient still has an on-going response (disease course is depicted in Figure 1).
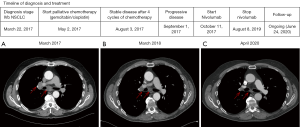
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Discussion
Here, we discuss a case of a metastatic NSCLC patient, progressing on first line chemotherapy, and presentation with a RET translocation, low PD-L1 and high TMB in 2017. The patient obtained durable benefit with ICI.
How can we put the data of this patient into perspective, with the currently available knowledge?
RET detection
The RET gene was identified in 1985, and encodes a receptor tyrosine kinase consisting of three domains: extracellular, transmembrane, and intracellular. RET activation can occur through mutations and fusions. Activation of RET leads to auto phosphorylation of RET and downstream cell signalling trough intracellular pathways such as mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3K)/AKT and Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathways which leads to uncontrolled cellular proliferation (11,12).
RET rearrangements were initially found in thyroid cancers, but also occur in 1–2% of NSCLC patients. In NSCLC, RET fusions are more commonly involved than single nucleotide variations (SNVs). In NSCLC at least 12 fusion partner genes have been identified so far, most common are the KIF5B-RET and CCDC6-RET (11). In contrast with our case, RET rearrangements are mainly observed in younger patients and never or light smokers (13,14). Consistent with our case, RET rearrangements are usually found in adenocarcinoma or in poorly differentiated tumours. Furthermore, RET point mutations and fusions are found in medullary and papillary thyroid carcinoma, spitzoid neoplasms, pancreatic cancer, ovarian cancer and colorectal cancers (15-17).
In general, different methods exist for detecting genomic rearrangements, each method having its advantages and drawbacks with respect to sensitivity, specificity, hands-on time and costs. Whereas ALK and ROS1 rearrangements are commonly detected using IHC and/or FISH, RET translocations cannot be adequately detected by IHC, as currently no validated antibody is available for diagnostic application (11). Based on the break-apart probe signal (‘classic pattern’), FISH allows detection of gene rearrangements, however, no fusion genes can be detected or specified. As illustrated in our study, in some cases a loss of the 5’ probe signal (centromere side) might be observed, while the 3’probe signal (telomere side) is maintained. Such probe pattern suggests the presence of a potential RET gene rearrangement or deletion, but does not clarify whether a RET gene fusion is present, resulting in an active fusion transcript (18). Moreover, with respect to the ROS1 translocation FISH, it was previously shown that the presence of isolated extra 3’ probe signal can lead to false positive FISH interpretation. By comparing ROS1 FISH with IHC and RNA sequencing, it was shown that in 10 cases FISH analyses indicated a translocation based on the presence of isolated 3’probe signal, whereas the IHC as well as RNA sequencing outcomes were negative (19). RNA sequencing allows detection of gene rearrangements that result in a fusion-transcript, and therefore is a reliable approach to characterize genomic rearrangements that are likely to result in oncogenic events. At the time of WGS sequencing in our case, the WGS bioinformatics pipeline was not suitable for detecting large genomic rearrangements, and therefore no such genomic alterations were reported. Currently, different commercial NGS RNA fusion gene panels exist (e.g., Archer FusionPlex, Illumina Trusight RNA panels, Oncomine Comprehensive assays, QuantideX NGS RNA assay), which in contrast to the sequencing of a complete transcriptome, allow detection of a limited number of target gene-transcripts in a relatively cost effective manner. RNA sequencing libraries can be generated based on hybrid capture enrichment, multiplex amplicons or anchored multiplex PCR, where sequencing methodologies differ in their ability to detect RNA fusion transcripts and characterize known and novel gene fusion partners (20). Yet, characterization of the specific gene fusion partner could be of significant value in near future, when highly selective RET inhibitors might become the new standard in treatment. As an example, with respect to crizotinib treatment in NSCLC patients with an EML4-ALK fusion, it was shown that even the specific variant of the EML4-ALK fusion transcript (variant 3/5 versus other EML4-ALK fusion transcripts) influenced progression free survival and overall survival (21).
The frame shift mutation in the RB1 gene [c.1768delT, p.(Cys590fs)], as detected in the DNA of our patient, could have therapeutic consequences when considering inclusion in ongoing RET-TKI trials, as RB1 functions downstream of RET, suppressing cell-cycle progression.
Treatment of RET rearranged NSCLC
Developing a TKI for treatment of RET rearranged patients has been challenging, as is described below.
Ret inhibitors
Initially, multi-target TKIs, such as vandetanib, cabozantinib, lenvatinib, alectinib, sunitinib, ponatinib, nintedanib, regorafenib and sorafenib were evaluated for the treatment of RET-rearranged NSCLC patients (22,23).
The first documented results of RET-TKI therapy were with cabozantinib in 2013 (n=3) (24). Two patients experienced a partial response (PR) and the third had prolonged stable disease (SD). The phase II trials in NSCLC, with cabozantinib (25), lenvatinib (26) and vandetanib (27,28) (Table 1), showed ORR of 16–53%, a median PFS of 4.5–7.3 months and a median OS of 9.9–11.6 months. For the other multi-kinase inhibitors used in the treatment of RET-rearranged NSCLC even more limited data is available. Unfortunately, multi-kinase inhibitors are associated with high rates of adverse effects (AEs) due to their anti-VEGFR (hypertension, proteinuria) and anti-EGFR activity (rash, diarrhoea). In 70% of the cases treatment discontinuation or dose reductions because of toxicity were necessary.
Table 1
| Author, year | Histology | Phase trial | RET inhibitor | Number of NSCLC patients | ORR % (95% CI) | mPFS, months (95% CI) | mOS, months (95% CI) |
|---|---|---|---|---|---|---|---|
| MKI’s | |||||||
| Drilon et al. 2016 | NSCLC | II | Cabozantinib | 26 | 28 (12 to 49) | 5.5 (3.8 to 8.4) | 9.9 (8.1 to not reached) |
| Yoh et al. 2017 (LURET) | NSCLC | II | Vandetanib | 19 | 53 (28 to 77) | 4.7 (2.8 to 8.5) | 11.1 (9.4 to not reached) |
| Hida et al. 2017 | NSCLC | II | Lenvatinib | 25 | 16 (N/A) | 7.3 (3.6 to 10.2) | Not evaluable (5.5 to not reached) |
| Lee 2017 | NSCLC | II | Vandetanib | 17 | 18 (N/A) | 4.5 (N/A) | 11.6 (N/A) |
| Selective TKI’s | |||||||
| Drilon et al. 2019 (LIBRETTO-001) | NSCLC, TC | I/II | Selpercatinib | 105 | 68% (58 to 76) | 18.4 (12.9–24.9) | N/A |
| Gainor et al. 2019 (ARROW) | NSCLC, TC, other | II | Pralsetinib | 120 | 58% (N/A) | N/A | N/A |
RET, rearranged during transfection; ORR, objective response rate; CI, confidence interval; mPFS, median progression free survival; mOS, median overall survival; NSCLC, non-small cell lung cancer; N/A, not available; TC, thyroid cancer.
The new selective RET inhibitors, selpercatinib (LOXO-292) and pralsetinib (BLU-667) are much more promising and showed impressive activity with a low percentage of severe AE (SAEs) in phase I/II trials. Selpercatinib is being investigated in the LIBRETTO-001 trial for RET rearranged solid tumours. Preliminary data of 105 NSCLC patients show an ORR of 68% (95% CI: 58–76) in the primary analysis and a median PFS of 18.4 months (95% CI: 12.9–24.9). Anti-RET activity was seen regardless of RET fusion partners, previous chemotherapy or cerebral metastasis. The ORR for cerebral metastasis was 91% (95% CI: 59–100). The tolerability was good with mostly grade 1–2 AEs (diarrhoea, dry mouth) with a discontinuation rate of 1.7% (17). Pralsetinib is being investigated in the ARROW study. Preliminary findings report an ORR of 58% (n=120), regardless of RET fusion partners and previous treatment of multi-kinase inhibitors. 40% of the patient had baseline brain metastases and pralsetinib led to a response of the brain metastasis in 7 out of 9 patients. Most of the AEs were grade 1–2 (increased AST/ALT, hypertension, constipation and fatigue) (29). RXDX-105 is a VEGFR-sparing, multikinase inhibitor with activity against RET and to a lesser degree against wild-type BRAF and BRAF V600E. Because RXDX-105 is VEGFR-sparing it has lower toxicity compared to other multikinase inhibitors, resulting in less dose modifications and/or discontinuation. In a phase I/Ib study ORR was 67% in those with non-KIF5B RET partners, while KIF5B RET fusions showed no response. Most common toxicities were fatigue, diarrhoea, hypophosphatemia and rash (30). Recently the FDA approved selpercatinib for the treatment of patients with metastatic RET fusion–positive NSCLC, metastatic RET-mutant medullary thyroid cancer (MTC) and metastatic RET fusion-positive thyroid cancer (31).
Although these selective RET inhibitors show promising results, acquired resistance will eventually occur and can limit long-term efficacy. RET solvent front mutations (G810R, G810S, G810C), evaluated in circulating tumour DNA (ctDNA), have recently been found to cause acquired resistance to selpercatinib and pralsetinib (23). Comparable with acquired EGFR and ALK mutations in TKI treated EGFR-mutated/ALK rearranged patients, these mutations were detected before clinical or radiographic progression (32). To overcome resistance to selpercatinib and pralsetinib, new inhibitors that have both activity against gatekeeper and solvent front mutations are being developed and investigated (33).
Ongoing or planned first line studies in RET rearranged NSCLC are for example the randomized phase III LIBRETTO-431 (NCT04194944, selpercatinib versus SoC platinum-doublet chemotherapy +/− pembrolizumab) and the randomized, open-label, phase III first AcceleRET Lung Study (NCT04222972, pralsetinib versus SoC platinum-doublet chemotherapy +/− pembrolizumab). The single arm phase II ALERT study (NCT03445000) is evaluating the activity of alectinib in pre-treated NSCLC patients. The single arm phase 2 LIBRETTO-321 study (NCT04280081) investigates the effectiveness of selpercatinib in patients with advanced solid tumours, including RET Fusion. The phase 1/2 study of TPX-0046 (NCT04161391), a RET/SRC inhibitor in solid tumours with a RET rearrangement is enrolling. A phase 1 study with RXDX-105 (NCT03784378) for the treatment of patients with NSCLC harbouring a RET Fusion and patients with ovarian cancer harbouring a BRAF mutation is planned but not recruiting yet. BOS172738 (NCT03780517) is being investigated in a phase I study including patients with NSCLC and Medullary Thyroid Cancer (MTC) with RET rearrangements. The LUNG-MAP Sub-Study, NCT04268550, is a phase II study evaluating the activity of selpercatinib in metastatic or recurrent NSCLC patients with RET Fusion. Cabozantinib (NCT01639508) is still being investigated in a phase II trial including NSCLC patients with RET fusion, ROS1 or NTRK Fusions or increased MET or AXL Activity. Last, cabozantinib is also being investigated in the phase II CRETA trial (NCT04131543) enrolling pre-treated, advanced RET-rearranged NSCLC patients.
RET-immunotherapy
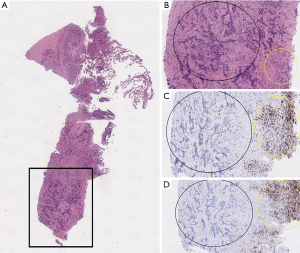
Limited data is available regarding the immune environment and ICI efficacy in RET positive NSCLC (Table 2) (8,23,34-36). In a retrospective study including 74 patients with RET rearranged lung cancer, the expression of PD-L1 (22C3 clone) was lower than 50% in 21 of 26 patients (81%), with a PD-L1 expression of 0% in 58% (n=15/26) cases and PD-L1 expression of 1–49% in 23% (n=6/26). In 44 patients, TMB was determined with MSK-IMPACT assay: median TMB was 1.75 mutations/Mb, which was significantly lower than the median TMB of RET wild-type NSCLCs, 5.27 mutations/Mb (n=3,631). The RET rearranged patients showed no response to ICI, and the median PFS was 3.4 months (34). In the six RET rearranged NSCLC patients included in the Immunotarget cohort, the median percentage of PD-L1 expression was 26 (range, 0–80%) (8). The ORR was only 6%, median PFS was 2.1 months. Another study included 785 patients with NSCLC who underwent surgery, all were tested for oncogenic drivers and PD-L1 expression. None of the six RET rearranged patients had a PD-L1 expression of more than 1% (37). A retrospective study of Guisier et al. showed 9 patients with RET-arrangements, with a PD-L1 expression of >50% in 2 patients (22%) and a negative PD-L1 status in 5 patients (56%). This study showed higher response rate in patients with a RET rearrangement (36). These limited data suggest that generally, patients with RET rearranged NSCLC have both low PD-L1 expression and low TMB, and do not respond well to ICI monotherapy, except in the small study of Guisier et al. Our patient indeed had a low PD-L1 level, but high TMB and obtained durable benefit with nivolumab. The high TMB could be related to his smoking status (100 pack years). Furthermore, high TMB is associated with ICI benefit (38), and this could be the explanation in our case too. To further evaluate the cancer immune environment in our patients, we evaluated the percentage of tumour infiltrating lymphocytes (TILs) in the sample obtained before start of nivolumab. TILs are associated with improved survival of NSCLC patients upon ICI treatment (39). However, the tumour tissue of our patient showed only a very low number of TILs (Figure 2). Detailed immune environment data are lacking for RET rearranged NSCLC. In general, it has been described that activated RET can activate the Wnt pathway which acts inhibitory on the recruitment of dendritic cells (17,40). Based on our case and the Guisier study, some patients with RET rearranged NSCLC respond well to ICI, and more data are needed to identify this special subset.
Table 2
| Author, type of study | RET+ patients (N) | PD-L1+ | TMB (median) and type of test used | ORR in RET | mPFS (months, 95% CI) | mOS (months, 95% CI) | mTTP (months, 95% CI) |
|---|---|---|---|---|---|---|---|
| Mazieres et al. (retrospective) | 16 | 6/16, mean 26% (0–80%) | N/A | 6% | 2.1 (1.3–4.7) | 21.3 (3.8–28) | N/A |
| Offin et al. (retrospective) | 74 | 0% in 58% (15/26); 1–49% in 23% (6/26); ≥50% in 19% (5/26) | 44 patients with known TMB (MSK-IMPACT); median TMB: 1.75 mut/Mb (range, 0–9.65) | 13 patients, 0% | 3.4 months (2.1–5.6) | OS compared between ICI naïve (n=46) and ICI (n=16): no difference (HR 1.4, 95% CI: 0.7–2.9) | N/A |
| Hegde et al. (retrospective, abstract only) | 72 (19 ICI, 53 non-ICI); 29 NSCLC | Available for 7 patients: 5/7 PD-L1 ≥1% | N/A | N/A | N/A | N/A | 10.5 (6.7–30.1); HR: ICI vs. non-ICI 1.73 RETf and 8.0 RETm |
| Guisier et al. (retrospective) | 9 | Positive in 33%; >50% in 22%; negative 56%; unknown in 11% | N/A | 37.5% | 7.6 (2.3 to NR) | NR (26.8 to NR) | N/A |
N, number; PD-L1, programmed death ligand 1; RET, rearranged during transfection; patients, patients; TMB, tumor mutational burden; ORR, objective response rate; mPFS, median progression free survival; CI, confidence interval; mOS, median overall survival; mTTP, median time to progression; N/A, not available; NR, not reached; ICI, immune checkpoint inhibitor; HR, hazard ratio; NSCLC, non-small cell lung cancer; RETf, RET fusions; RETm, RET mutations.
The role of ICI combined with chemotherapy in RET rearranged NSCLC is not clear, and hopefully on-going clinical trials will shed some light. For example, the POSEIDON trial (NCT04322591) is evaluating chemotherapy with or without a PD-1 antibody in RET fusion positive NSCLC. Furthermore, the LIBRETTO-431 and AcceleRET Lung have in their SoC arm the option to combine platinum-doublet chemotherapy with pembrolizumab.
Conclusions
RET rearranged NSCLC has been difficult to treat for years with disappointing results of MKIs. However, the new selective RET TKIs are very promising in early phase clinical trials with high ORR and durable responses, combined with low toxicity. Phase III trials are on-going. Tumours with RET fusions and mutations often express low PD-L1 and have low TMB and ICI monotherapy data are disappointing in this patient population. It is not clear yet whether combination therapies with chemotherapy and ICI are effective in RET rearranged NSCLC.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Precision Cancer Medicine for the series “Precision Oncology Tumor Board”. The article was sent for external peer review organized by the Guest Editor Dr. Balazs Halmos and the editorial office.
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/pcm-20-26
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pcm-20-26). The Series “Precision Oncology Tumor Board” was commissioned by the editorial office without any funding or sponsorship. LELH reports (none related to current manuscript, outside of current manuscript) research funding Roche Genentech, Boehringer Ingelheim, AstraZeneca (all institution), advisory board: Boehringer, BMS, Eli Lilly, Roche Genentech, Pfizer, Takeda, MSD, Boehringer Ingelheim (all institution), speaker: MSD, travel/conference reimbursement: Roche Genentech, BMS (self); mentorship program with key opinion leaders: funded by AstraZeneca; fees for educational webinars: Quadia (self), interview sessions funded by Roche Genentech (institution), local PI of clinical trials: AstraZeneca, Novartis, BMS, MSD /Merck, GSK, Takeda, Blueprint Medicines, Roche Genentech. LELH serves as an unpaid editorial board member of Precision Cancer Medicine from Dec 2018 to Nov 2020. EJMS reports (none related to current manuscript, outside of current manuscript) research funding: Amgen, AstraZeneca, advisory board: Bayer, Janssen-Cilag, presentation: BMS. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv192-237. [Crossref]
- EMA Pembrolizumab. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/keytruda. Access date 05/08/2020
- FDA Pembrolizumab. Available online: https://www.drugs.com/history/keytruda.html. Access date 05/08/2020
- Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819-30. [Crossref] [PubMed]
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Remon J, Hendriks LE, Cabrera C, et al. Immunotherapy for oncogenic-driven advanced non-small cell lung cancers: Is the time ripe for a change? Cancer Treat Rev 2018;71:47-58. [Crossref] [PubMed]
- Nagahashi M, Sato S, Yuza K, et al. Common driver mutations and smoking history affect tumor mutation burden in lung adenocarcinoma. J Surg Res 2018;230:181-5. [Crossref] [PubMed]
- Mazieres J, Drilon A, Lusque A, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol 2019;30:1321-8. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- van der Velden DL, Hoes LR, van der Wijngaart H, et al. The Drug Rediscovery protocol facilitates the expanded use of existing anticancer drugs. Nature 2019;574:127-31. [Crossref] [PubMed]
- Ferrara R, Auger N, Auclin E, et al. Clinical and Translational Implications of RET Rearrangements in Non-Small Cell Lung Cancer. J Thorac Oncol 2018;13:27-45. [Crossref] [PubMed]
- Ackermann CJ, Stock G, Tay R, et al. Targeted Therapy For RET-Rearranged Non-Small Cell Lung Cancer: Clinical Development And Future Directions. Onco Targets Ther 2019;12:7857-64. [Crossref] [PubMed]
- O'Leary C, Xu W, Pavlakis N, et al. Rearranged During Transfection Fusions in Non-Small Cell Lung Cancer. Cancers (Basel) 2019;11:620. [Crossref] [PubMed]
- Bronte G, Ulivi P, Verlicchi A, et al. Targeting RET-rearranged non-small-cell lung cancer: future prospects. Lung Cancer (Auckl) 2019;10:27-36. [Crossref] [PubMed]
- Gozgit JM, Chen TH, Song Y, et al. RET fusions observed in lung and colorectal cancers are sensitive to ponatinib. Oncotarget 2018;9:29654-64. [Crossref] [PubMed]
- Gainor JF, Shaw AT. Novel targets in non-small cell lung cancer: ROS1 and RET fusions. Oncologist 2013;18:865-75. [Crossref] [PubMed]
- Drilon A, Oxnard G, Wirth L, et al. Registrational Results of LIBRETTO-001: A Phase 1/2 Trial of LOXO-292 in Patients with RET Fusion-Positive Lung Cancers. J Thorac Oncol 2019;14:S6-7. [Crossref]
- Huang RSP, Smith D, Le CH, et al. Correlation of ROS1 Immunohistochemistry With ROS1 Fusion Status Determined by Fluorescence In Situ Hybridization. Arch Pathol Lab Med 2020;144:735-41. [Crossref] [PubMed]
- Heydt C, Ruesseler V, Pappesch R, et al. Comparison of in Situ and Extraction-Based Methods for the Detection of ROS1 Rearrangements in Solid Tumors. J Mol Diagn 2019;21:971-84. [Crossref] [PubMed]
- Bubendorf L, Buttner R, Al-Dayel F, et al. Testing for ROS1 in non-small cell lung cancer: a review with recommendations. Virchows Arch 2016;469:489-503. [Crossref] [PubMed]
- Su Y, Long X, Song Y, et al. Distribution of ALK Fusion Variants and Correlation with Clinical Outcomes in Chinese Patients with Non-Small Cell Lung Cancer Treated with Crizotinib. Target Oncol 2019;14:159-68. [Crossref] [PubMed]
- Farago AF, Azzoli CG. Beyond ALK and ROS1: RET, NTRK, EGFR and BRAF gene rearrangements in non-small cell lung cancer. Transl Lung Cancer Res 2017;6:550-9. [Crossref] [PubMed]
- El Osta B RS. RET Fusion: Joining the Ranks of Targetable Molecular Drivers in Non-Small Cell Lung Cancer. JTO Clinical and Research Reports 2020. Available online: https://doi.org/
10.1016/j.jtocrr.2020.100050 - Drilon A, Wang L, Hasanovic A, et al. Response to Cabozantinib in patients with RET fusion-positive lung adenocarcinomas. Cancer Discov 2013;3:630-5. [Crossref] [PubMed]
- Drilon A, Rekhtman N, Arcila M, et al. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: an open-label, single-centre, phase 2, single-arm trial. Lancet Oncol 2016;17:1653-60. [Crossref] [PubMed]
- Hida T, Velcheti V, Reckamp KL, et al. A phase 2 study of lenvatinib in patients with RET fusion-positive lung adenocarcinoma. Lung Cancer 2019;138:124-30. [Crossref] [PubMed]
- Yoh K, Seto T, Satouchi M, et al. Vandetanib in patients with previously treated RET-rearranged advanced non-small-cell lung cancer (LURET): an open-label, multicentre phase 2 trial. Lancet Respir Med 2017;5:42-50. [Crossref] [PubMed]
- Lee SH, Lee JK, Ahn MJ, et al. Vandetanib in pretreated patients with advanced non-small cell lung cancer-harboring RET rearrangement: a phase II clinical trial. Ann Oncol 2017;28:292-7. [Crossref] [PubMed]
- Gainor JF, Lee DH, Curigliano G, et al. Clinical activity and tolerability of BLU-667, a highly potent and selective RET inhibitor, in patients (pts) with advanced RET-fusion plus non-small cell lung cancer (NSCLC). Available online: https://www.blueprintmedicines.com/wp-content/uploads/2019/05/Blueprint-Medicines-ASCO-2019-BLU-667-NSCLC-Presentation.pdf
- Drilon A, Fu SQ, Patel MR, et al. A Phase I/Ib Trial of the VEGFR-Sparing Multikinase RET Inhibitor RXDX-105. Cancer Discovery 2019;9:384-95. [Crossref] [PubMed]
-
Selpercatinib FDA 2020 . Available online: https://www.drugs.com/history/retevmo.html - Herbreteau G, Vallee A, Charpentier S, et al. Circulating free tumor DNA in non-small cell lung cancer (NSCLC): clinical application and future perspectives. J Thorac Dis 2019;11:S113-26. [Crossref] [PubMed]
- Solomon BJ, Tan L, Lin JJ, et al. RET Solvent Front Mutations Mediate Acquired Resistance to Selective RET Inhibition in RET-Driven Malignancies. J Thorac Oncol 2020;15:541-9. [Crossref] [PubMed]
- Offin M, Guo R, Wu SL, et al. Immunophenotype and Response to Immunotherapy of RET-Rearranged Lung Cancers. JCO Precis Oncol 2019; [Crossref] [PubMed]
- Hegde A, Huang L, Liu S, et al. Responsiveness to immune checkpoint inhibitors in RET dependent cancers. Atlanta, USA: Annual American Association for Cancer Research (AACR) Meeting, Abstract #4497, April 30, 2019.
- Guisier F, Dubos-Arvis C, Vinas F, et al. Efficacy and Safety of Anti-PD-1 Immunotherapy in Patients With Advanced NSCLC With BRAF, HER2, or MET Mutations or RET Translocation: GFPC 01-2018. J Thorac Oncol 2020;15:628-36. [Crossref] [PubMed]
- Liang W, Guo M, Pan Z, et al. Association between certain non-small cell lung cancer driver mutations and predictive markers for chemotherapy or programmed death-ligand 1 inhibition. Cancer Sci 2019;110:2014-21. [PubMed]
- Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. [Crossref] [PubMed]
- Brambilla E, Le Teuff G, Marguet S, et al. Prognostic Effect of Tumor Lymphocytic Infiltration in Resectable Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:1223-30. [Crossref] [PubMed]
- Castellone MD, Melillo RM. RET-mediated modulation of tumor microenvironment and immune response in multiple endocrine neoplasia type 2 (MEN2). Endocr Relat Cancer 2018;25:T105-19. [Crossref] [PubMed]
Cite this article as: Dursun S, Theunissen TEJ, Li X, Speel EJM, Hendriks LEL. Prolonged immune checkpoint inhibitor response of a patient with a RET rearranged non-small cell lung cancer and high tumour mutational burden: case report and review of the literature. Precis Cancer Med 2020;3:22.

