
How to recognize and manage hyper-progression and pseudo-progression during immune checkpoint blockade in non-small cell lung cancer
Introduction
The advent of immune checkpoint blockers (ICB) for the treatment of advanced non-small cell lung cancer (NSCLC) has generated, besides indisputable benefits, new clinical challenges for physicians, aiming to provide every treated patient with the best outcomes in terms of both length and quality of survival. The arising toxicities in the spectrum of auto-immunity require awareness and a prompt management (1), while the need for a correct patient selection has prompted the development of several pathological, clinical and molecular biomarkers to predict immunotherapy benefits (2). Of most interest, novel clinic-radiological features associated to ICB treatment have been recognized in solid malignancies and in NSCLC. Indeed, hyperprogression and pseudo-progression question the respective paradigms that an anti-cancer agent should not foster tumor growth and that tumor response should be accompanied by disease dimensional decrease. Owing to the spread of immunotherapy strategies, clinicians and radiologists involved in the care of cancer patients receiving ICB should be aware of these patterns, whose recognition and management may result crucial for improving patients outcomes.
Hyperprogressive disease: prevalence and clinical predictors
Programmed cell death protein 1 (PD-1) and programmed cell death ligand 1 (PD-L1) inhibitors, restoring the immune response against cancer cells, have significantly prolonged survival compared to chemotherapy, with a five-year survival rate of 15-20% in pretreated patients (3). However, a rapid progression, upon immune checkpoint blockade, known as hyperprogressive disease (HPD), has recently been reported in a relatively small subgroup of NSCLC patients (4,5). The existence of HPD was initially suggested by the crossing of Kaplan Mayer survival curves in the CheckMate 057 (6) study and in the cohort 1 of Atlantic trial including EGFR-mutated and ALK-rearranged patients (7).
Since the beginning of 2017, HPD has been described in several studies (Table 1). Firstly, Champiat et al. reported an acceleration of tumor growth in 12 (9%) out of 131 evaluable cancer patients treated with anti-PD-1/PD-L1 agents within phase I trials. In this study, tumor growth rate, representing the variation of tumor volume during time, was computed before (TGR ref) and during (TGR exp) anti-PD-1/PD-L1 therapy. HPD was defined as disease progression by RECIST and a 2-fold increase in the TGR ratio (TGR exp/TGR ref). HPD was not associated with either specific tumor type or higher baseline tumor burden, while it was significantly related to higher age and worse survival outcome (8). Of note, no cases of HPD in lung cancer patients were reported in the study by Champiatet al. (out of 13 treated patients).
Table 1
| Author | Prevalence and study setting | Agent and treatment line | Criteria for HPD | Clinical or biological predictors of HPD |
|---|---|---|---|---|
| Champiat; Clin Cancer Res 2017 (8) | 9% (12/131); phase I trial | Anti-PD-1 or anti-PD-L1 monotherapy. Different treatment lines | RECIST progression after first evaluation and ≥2-fold increase of the TGR between the reference and the experimental periods | ≥65 years old |
| Kato; Clin Cancer Res 2017 (9) | 6% (6/102); stage IV cancer patients | Anti-PD-1, anti-PD-L1, anti-CTLA4 or other investigational. Different treatment lines | TTF <2 months, >50% increase in tumor burden compared with baseline preimmunotherapy imaging, and >2-fold increase in progression pace | MDM2/MDM4 and EGFR alterations. DNMT3A alterations (multivariate analysis) |
| Saâda-Bouzid; Ann Oncol 2017 (10) | 29% (10/34); head and neck cancer | Anti-PD-1 or anti-PD-L1 monotherapy. Different treatment lines | TGK (variation of largest diameter of target lesions per unit of time) ratio ≥2 | Locoregional recurrence |
| Singavi; ESMO 2017 (11) | 5 patients; solid tumours (oesophageal, lung, renal cell carcinoma) | Anti-PD-1 agents. Different treatment lines | Progression at first restaging on immunotherapy, increase in tumor size >50%, >2-fold increase in TGR | MDM2/MDM4, EGFR, amplifications on 11q13 (CCND1, FGF3, FGF4, FGF19) |
| Matos Garcia; ASCO 2018 (12) | 15% (33/214); solid tumors | Anti-PD-1 or anti-PD-L1 as monotherapy or combo with other agents. Different treatment lines | TTF <2 months and: (I) increase of ≥40% in target tumor burden compared to baseline or (II) increase ≥20% in target tumor burden plus multiple new lesions | None |
| Kim; Ann Oncol 2019 (13) | 55 (21%) according TGK (14); 54 (20%) according TGR ratio (6); 98 (37%) according TTF <2 months; NSCLC | Anti-PD-1 or anti-PD-L1 monotherapy. Different treatment lines | defined by tumor growth kinetic ratio ≥2, TGR ratio ≥2 or TTF <2 months | Lower frequency of circulating effector memory (CD45RA-CCR7-) CD8 T-cells, and higher frequency of severely exhausted (TIGIT+PD1+) CD8 T-cells |
| Kim; ASCO 2018 (15) | 17% (37/220); NSCLC | Single agent immunotherapy. Line of therapy not specified | defined by tumor growth kinetics on prior versus immunotherapy (details not provided) | None |
| Ferrara; JAMA Oncol (11) | 14% (56/406); NSCLC | Anti-PD-1 or anti-PD-L1 as monotherapy or in combination with other agents. Different treatment lines | Disease progression at the first evaluation with >50% change in TGR | >2 metastatic sites prior to immunotherapy |
| Kanjanapan; Cancer 2019 (16) | 7% (12/182); solid tumors | Anti-PD-1, anti-PD-L1, anti-CTLA4 or other investigational drugs as monotherapy or combos. Different treatment lines | RECIST 1.1 progression at the first on-treatment scan and ≥2-fold increase in TGR between experimental and reference period | Female gender |
| Lo Russo; Clin Cancer Res 2019 (5) | 26% (39/187); NSCLC | Anti-PD-1 or anti-PD-L1 as monotherapy or combo with anti-CTLA4. Different treatment lines | TTF <2 months, increase ≥50% in the sum of target lesions major diameters, appearance of at least two new lesions in an organ already involved, spread of the disease to a new organ, ECOG PS worse ≥ 2 during the first 2 months. HPD on the basis of 3 concomitant out of the 5 possible criteria | Clustered macrophages with epithelioid morphology and co-localization of CD163, PD-L1, and CD33 markers (defined as complete phenotype) |
| Kamada; PNAS 2019 (17) | 11% (4/37); gastric cancer | Nivolumab monotherapy. Different treatment lines | TTF <2 months; >50% increase in tumor burden compared with pre-treatment imaging, and >2-fold increase in progression pace | PD-1+ effector (CD45RA-) T-regulatory (Foxp3+ CD4+) cells. 1 patient with HPD had MDM2 amplification |
| Sasaki; Gastric Cancer 2019 (14) | 21% (13/62); gastric cancer | Nivolumab monotherapy. Different treatment lines | >2-fold increase in TGR | Absolute neutrophil count, C-reactive protein levels, baseline tumour burden, presence of liver metastases and worse ECOG performance status were associated with HPD |
| Xiong; iScience 2018 (18) | 2 patients; solid tumours (renal cell and oesophageal carcinoma) | Anti-PD-1 monotherapy. Different treatment lines | as by Kato et al. | TSC2, VHL mutations, transcriptional upregulation of oncogenic pathways, including IGF-1, ERK/MAPK, PI3K/AKT, and TGF-ß. Elevated innate lymphoid cells ILC3 |
| Ratner; New Engl J Med 2018 (19) | 3 patients; adult T-cell leukemia lymphoma patients | Nivolumab monotherapy. Details not provided | Details not provided | None |
HPD, hyperprogressive disease; TGR, tumor growth rate; TTF, time to treatment failure; TGK, tumor growth kinetic; NSCLC, non-small cell lung cancer; ECOG PS, Eastern Cooperative Oncology Group Performance Status.
Using similar TGR criteria, Kanjanapan et al. (16) and reported HPD in 7% of 352 cancer patients in phase I trials (with a higher proportion of HPD in female patients) testing ICB. Sasaki et al. (14) described HPD in 21% of 62 gastric cancer patients (with a significant correlation between HPD and absolute neutrophil count, C-reactive protein levels, baseline tumour burden, presence of liver metastases and performance status) receiving anti-PD-1 agents. HPD was reported also in 10 (29%) out of 34 patients with recurrent and/or metastatic head and neck squamous cell carcinoma treated with PD-1/PD-L1 inhibitors by Saâda-Bouzid et al. (10). In this study, HPD was defined as an increase of at least 2-fold in tumor growth kinetics (TGK) (which measures the variation of the sum of the largest diameters of target lesions per unit of time), compared to TGK before immunotherapy. HPD was not associated with local or distant recurrence, but it was significantly related to regional recurrence.
Moving to the specific setting of advanced NSCLC, HPD was reported in 13.8% of 406 cases treated with single agent PD-1/PD-L1 inhibitors in a French multicentric study by Ferrara et al. (4). In this study, HPD was defined as disease progression by RECIST and >50% increase in the TGR variation during ICI compared to TGR before immunotherapy (TGR exp-TGR ref >50%). HPD significantly correlated with more than two metastatic sites before ICI compared to non-HPD (62.5% vs. 42%, P=0.006) and worse overall survival compared to conventional disease progression [3.4 months (95% CI: 2.8–7.5) vs. 6.2 months (95% CI: 6.3–7.9), P=0.003]. This study was the largest multicentric analysis exploring HPD in a dedicated NSCLC population, and included also a control chemotherapy cohort. Interestingly, HPD was described only in three (5%) of 59 NSCLC patients treated with single agent chemotherapy in very advanced lines, suggesting that HPD may be a new pattern specifically related to ICI.
Recently, Kim et al. described HPD (defined by volume-based growth kinetics) in 17% of 220 advanced NSCLC patients after single-agent ICI. HPD was associated with a significantly lower median PFS (1.2 vs. 4.1 months, P<0.001) and median OS (7.1 vs. 15.9 months, P=0.09) and did not correlate with patients’ clinical characteristics (15).
Kato et al. (9) investigated potential genomic markers associated to HPD in 102 stage IV cancer patients treated with ICB. HPD was defined as a time to treatment failure (TTF) <2 months, increase of more than 50% in tumor burden compared with pre-immunotherapy imaging and a >2-fold increase in progression pace (progression rate in the first two months of immunotherapy/progression rate in the previous 2 months before starting immunotherapy). Overall, HPD was identified in six patients (~6%): four patients (NSCLC, endometrial carcinoma, urothelial carcinoma, triple negative breast cancer) had MDM2 amplifications and two NSCLC patients had EGFR mutations.
In addition, MDM2/MDM4, EGFR and 11q13 (including CCND1, FGF3, FGF4, FGF19) amplifications were found in two (50%), one (25%) and three (75%) of four patients with HPD upon ICB and available next generation sequencing (NGS) data. The only NSCLC patient with HPD had MDM2, FGF3, FGF4 and FGF19 amplifications. In this study, HPD criteria were progression at first restaging on ICI, increase in tumor size >50% and >2-fold increase in TGR (11).
Most of research groups have included pre-baseline tumour assessment and defined HPD according to a substantial change in pace of tumour growth, in order to avoid that rapid progression to ICB may simply reflect the natural history of a very aggressive disease (20). However, pre-baseline imaging may not be always available in first line setting. Furthermore, both RECIST and iRECIST criteria, only evaluate changes in tumor size, understating the real tumor burden (non-target lesions, such as bone metastases, lymphangitis, pleural and peritoneal effusions). In this regard, two research groups defined HPD with different criteria not including the assessment of pre-baseline tumor growth.
Lo Russo et al. (5) defined patients with HPD those who fulfilled at least three of the following clinical/radiological criteria: (I) spread of the disease to a new organ between baseline and first radiologic evaluation; (II) increase ≥50% in the sum of target lesions major diameters between baseline and first radiologic evaluation; (III) appearance of at least two new lesions in an organ already involved between baseline and first radiologic evaluation; (IV) TTF <2 months; (V) clinical deterioration with decrease in ECOG performance status ≥2 during the first two months of treatment. HPD was identified in 39 (25.7%) of 152 NSCLC patients and was characterized by a significantly worse OS compared to non-HPD patients [4.4 months (95% CI: 3.4–5.4) vs. 17.7 months (95% CI: 13.4–24.1)].
Matos et al. (12) used a similar definition (TTF <2 months and increase in measurable lesions of >10 mm plus the following: (I) increase of ≥40% in target tumour burden compared to baseline; or (II) increase of ≥20% in target tumour burden plus multiple new lesions). Among 214 patients with solid tumours treated ICB (53% in combinations with other ICB), they found 33 cases (15%) of HPD. HPD was not associated with clinical characteristics and correlated with poor OS [4.8 months (95% CI: 3.4–7.3 months) vs. 8.7 months (95% CI: 6.3–10.2 months) in non-HPD progressor group (HR =1.87; 95% CI: 1.1–3.3; P=0.03)]. Recently, a similar percentage (13.1–14.3%) of HPD was found in 135 NSCLC treated with ICB according to one-dimensional or volumetric criteria. However, the discordance rate was of 16.3% and the volumetric definition was the only one correlated with a worse overall survival (21).
Finally, the definitions of fast progression (FP, ≥50% increase in the sum of long diameters within six weeks from baseline) and early deaths (ED, death due to disease progression within 12 weeks from the start of ICB) were proposed as surrogate of HPD and reported in 4.7% and 5.6% respectively of NSCLC patients treated with second or third line atezolizumab in the phase III OAK trial (22). However, the exact overlap between FP, ED and HPD is not evaluable in clinical trials due to the lack of collected pre-baseline imaging and in a recent panel session at the American Association for Cancer Research Annual Meeting 2019 in Atlanta, panelists unanimously concurred that the collection of pre-baseline scans in clinical trials should be standard in the future in order to properly assess HPD (23).
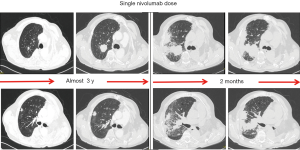
Figure 1 depicts an emblematic case of HPD in an advanced, pretreated NSCLC patient.
Potential underlying mechanisms of hyperprogressive disease
Up to now, several mechanisms of HPD such as T-regulatory cell expansion, T-effector cell exhaustion, modulation of pro-tumorigenic immune subset, aberrant inflammation and oncogenic pathway activation have been proposed (24), and both translational and in vivo studies have tried to identify a common underlying biology for HPD phenomenon. In post-therapy tumors from patients with esophageal and renal cell carcinoma experiencing HPD during ICB, somatic mutations were found in tumor suppressor genes such as TSC2 and VHL, along with transcriptional up-regulation of oncogenic pathways, including IGF-1, ERK/MAPK, PI3K/AKT, and TGF-ß (18). However, as for MDM2/4 amplification and EGFR mutations, how these molecular alterations could mediate an acceleration of tumor growth during ICB is currently unknown. Similarly, concomitant LKB1 and KRAS mutations were found in 3 (18%) out of 16 NSCLC patients experiencing HPD according to a volumetric definition (21).
A potential explanation may be found in the tumor suppressive role of PD-1, as reported in human T-cell lymphoma, where PD-1 seemed to down-regulate PI3K intracellular signaling (25,26). In this regard, a rapid progression was described in the first three patients with adult T cell leukemia after one single dose of nivolumab in a phase II trial (19).
The putative role of both innate and adaptive immunity in driving HPD has been recently investigated. Lo Russo et al. (5) identified clustered macrophages with epithelioid morphology and co-localization of CD163, PD-L1, and CD33 markers (defined as complete phenotype) as related to HPD in NSCLC patients. In addition, murine models and patients derived xenografts suggested that anti-PD1 antibody may drive HPD through reprogramming M2 immunosuppressive macrophages via Fc-Fc-receptor signal. Other innate immune cells such as neutrophils may also play a role in HPD, and high neutrophil lymphocyte ratio correlated both with poor survival outcome (27) and HPD (21) in NSCLC upon ICB.
HPD could also be mediated through the involvement of immunosuppressive T cells. In fact, Kamada et al. (17) reported an enhanced proliferation of PD-1+ T-regulatory cells in two gastric cancer patients with HPD during ICB. Tissue samples from these patients showed increased Ki67+ effector (CD45RA- Foxp3+ CD4+) T-regulatory cells compared to non-HPD patients. In vitro and in vivo models confirmed that PD-1 deficient T-regulatory cells are highly proliferative and immunosuppressive, supporting the hypothesis that PD-1 blockade besides releasing the brakes of CD8+ T cells against tumor may also paradoxically increase immune suppression in the tumor microenvironment through the involvement of T-regulatory cells.
Finally, pre-existing systemic immunity could also play a role in HPD as recently reported by Kim et al. In particular, lower circulating effector memory (CCR7- CD45RA-) CD8+ T-cells and higher severely exhausted (TIGIT+PD1+) CD8+ T-cells before ICI were found in HPD compared to non-HPD progressors NSCLC patients (13).
Pseudo-progression in NSCLC
Before the routine availability of ICB, oncological community dealt with pseudo-progression (pseudoPD) almost exclusively in the field of neuro-oncology, where radiation-induced damages in the tumor microenvironment, engendering inflammation, mimic uncontrolled tumor growth (28). Somewhat similarly with regard to ICB administration, the observation of pseudoPD features (initial tumor growth followed by dimensional decrease and actual disease response) depends on ICB mechanisms of action. Anti-PD-1/PD-L1 agents, as well as anti-CTLA-4 ones, release immune phenotypes, especially T lymphocytes, against cancer cells, acting thus far not directly against these latter. The indirect way ICB act against tumor is indeed through the recruitment of effective anticancer immune cells within tumor lesion, that as a consequence may undergo apparent increase in dimension, while malignant elements are actively being eliminated (29).
The first recognition of ICB-related pseudoPD in melanoma, the first tumor type in which ICB administration turned out to be effective, prompted the definition of novel criteria to be adopted for cancer patients undergoing immunotherapy (immune related response criteria, irRC) (30). Further modifications of these criteria, moving from the dimensional lesion evaluation of irRC, brought towards irRECIST and, finally, to immune RECIST (iRECIST) (31,32). The most relevant feature of these latter is represented by the introduction of the “immune-unconfirmed progression”, suggesting ICB can be maintained after the first radiologic evidence of tumor progression, conceiving a potential subsequent of delayed disease response.
Looking at clinical trials, up to 10% and 7% respectively of melanoma and renal cell carcinoma patients receiving ICB underwent pseudoPD. The occurrence of pseudoPD in NSCLC patients is less frequent (up to 3%) (22,33-37), reaching higher proportion if initial disease progression according to RECIST is followed by stabilization (and not by disease response, as in “conventional” pseudoPD). Considering the absolute number of cases undergoing ICB and the relevance of the clinical decision to be taken at the moment of the first sign of disease progression, pseudoPD is still a relevant issue in NSCLC. Considering the relative rare occurrence of pseudoPD during ICB in NSCLC patients, an extensive use of iRECIST for the daily clinical practice does not seem to be justified. Considering that no reliable markers of progression are available, careful evaluation of patients’ disease related symptoms and performance status, together with radiological evaluation, should guide physicians’ choices and help to better recognize atypical patterns of response such as pseudoPD (38).
Recognizing and managing pseudo-progression
Tables 2 and 3 gather several clinical information regarding cohorts (35,37,39) and case reports (40-60), respectively, of pseudoPD during ICB in NSCLC. A predisposition towards pseudoPD cannot be envisaged owing to tumor histology, smoking history and PD-L1 expression. The strong representation of nivolumab as the treatment received by patients who experienced pseudoPD is likely to be ascribed to its wider administration, especially in the first years since ICB availability, compared to other agents.
Table 2
| Reference | Patients | PseudoPD | Time-to-pseudoPD | PseudoPD sites | Outcomes |
|---|---|---|---|---|---|
| Katz; J Thorac Oncol 2018 (35) | 228 | 3 (2%) | < 3 months | Two: primary tumor | 2 out of 3 patients alive (3 years since nivolumab beginning) |
| One: new lung lesions | |||||
| Fujimoto; J Thor Oncol 2019 (37) | 542 | 14 (3%) | Median 1 month | All known lesions with additional new lesions in 3 cases | mPFS after pseudoPD: 7.3 months; mPFS of typical response: NR (P<0.001) |
| Hendriks; J Thorac Oncol 2019 (39) | 255 with brain metastases | 2 (0.8%) brain pseudoPD | NA | Known and new brain lesions | NA |
NA, not available; mPFS, median progression-free survival; NR, not reached.
Table 3
| Reference | Istology | PD-L1 IHC | Smoking history | Drug, line | Time-to-pseudoPD; clinical/imaging | PseudoPD styles already known? | Sites pseudoPD | ICB continuation | PFS | Pathologic evidence |
|---|---|---|---|---|---|---|---|---|---|---|
| Doherty; J Thorac Oncol 2015 (40) | Adeno | Positive | + | Anti-PD-1, II | 3 months; brain imaging | Yes | Brain | No | 14+ months | Necrotic tissue |
| Ito; J Thorac Oncol 2016 (41) | Pleomorphic | Positive | + | Nivo, VII | 7 days; clinical | Yes | Nodes | Yes | 16+ weeks | NA |
| Kolla; J Immunother Cancer 2016 (42) | Adeno | NA (EGFR mut) | − | Nivo, IV | Before week 8; clinical | Yes | Pleuro/pericardial effusions | No | 4 months | Pleural and pericardial cytologies positive for malignancy. Lymphocytes 30% of cells. |
| Tanizaki; Lung Cancer 2016 (43) | Adeno | NA | + | Nivo, II | 6 weeks; imaging | Yes | Liver | Yes | 7+ months | At regression: fibrotic tissue with no residual tumor cells, CD3+ lymphocytes infiltration |
| Adeno | NA | − | Nivo, II | 6 weeks; imaging | Yes | Liver | Yes | 6+ months | NA | |
| Curioni-Fontecedro; Ann Oncol 2017 (44) | Non-squamous | NA | NA | Nivo, II | 6 cycles; PET | No | Nodes, pleura, intestine and bones | Yes | NA | N: normal lymphocytes, no granuloma, no malignant cells. 60% CD3+ T lymphocytes and 30% CD19+. Polyclonal B lymphocytes |
| Izumida; BMJ Case rep 2017 (45) | Adeno | NA | NA | Nivo, VI | 2 months; imaging | Yes | T and nodes | Resumed at further PD, no response then | 5 months | NA |
| Kato; ESMO Open 2017 (46) | Adeno | NA | + | Nivo, VIII | 4 days; clinical | Yes | T and perilesional GGO | Resumed at further PD with similar behavior | 7+ months | NA |
| Sarfaty; Medicine (Baltimore) 2017 (47) | Squamous | NA | + | Nivo, III | 1 week; clinical, PET+ | Yes | Subcutaneous | Yes | NA | NA |
| Nishino; Clin Cancer Res 2017 (48) | Adeno | NA | NA | Nivo, NA | 1.4 months; imaging | Yes | Lung | Yes | 8.8 months (late PR) | NA |
| Hochmair; Lung Cancer 2017 (49) | Adeno | 90% | NA | Pembro, II | 2 months; clinical | Yes | T | Yes | 7+ months | Pleural effusion: highly PD-L1-expressing tumor cells |
| Adeno | 90% | NA | Pembro, II | 5 weeks; clinical | Yes + new | T, lung, pleaural and pericardial effusions, liver | Yes | 15+ months | ||
| Melian; Thorac Cancer 2018 (50) | NSCLC NOS | < 1% | + | Nivo + Ipi, I | 1 week; clinical | Yes | Brain | Yes | NA | NA |
| NSCLC NOS | < 5% | + | Nivo + Ipi, II | 1 week; clinical | Yes | Brain | Yes | 10+ cycles | NA | |
| Rocha; J Thorac Oncol 2018 (51) | Squamous | 100% | + | Nivo, II | 5 cycles; imaging | Yes | Liver | Yes | NA | Tumor necrosis. CD4+, CD8+, CD103+, CD68+ increase |
| Swami; J Thorac Oncol 2018 (52) | Squamous | 1-2% | + | Nivo, II | 2 months; clinical | Yes | Brain | Yes | 1.5+ years | NA |
| Yamaura; J Thorac Oncol 2018 (53) | Adeno | 95% | + | Pembro, II | 1 week; clinical | Yes | Lung GGO and nodes | Yes | NA | NA |
| Yoshimura; Clin Case Rep 2018 (54) | Pleomorphic | 80% | + | Nivo, V | 6 cycles; imaging | Yes | Lung and pleural effusion | Yes | 8 months+ | Pleural effusion: no tumor cells, predominantly lymphocytes |
| Laurie; Curr Oncol 2019 (55) | Adeno | NA | NA | Nivo, II | Several months; imaging | Yes | Liver and nodes | Yes | 8 months since pseudoPD | NA |
| Kim; Mol Clin Oncol 2019 (56) | Non-squamous | NA | NA | Pembro, II | 4 months; clinical | Yes | Small bowel | Yes | NA | Tumor cells present. Lymphocytes, especially CD8+ |
| Asai; Immunotherapy 2019 (57) | Squamous | 90% | + | Nivo, II | 7 days; clinical | yes | Pericardial effusion, T and nodes | Yes | 7 months | Tumor cells on pericardiocentesis |
| Kogure; J Thorac Oncol 2019 (58) | Adeno | 70-80% | + | Pembro, I | 2 days; clinical (CRS) | Yes | T and nodes | Yes | NA | No |
| Mashuiro; J Thorac Oncol 2019 (59) | Adeno | ≥ 50% | − | Pembro, I | Few days; clinical | Yes | T cavitation | Yes | 5+ months | Intense infiltration of CD3+ lymphocytes. No cancer cells, infection, organizing pneumonia, granulomatous inflammation |
| Suyanto; Case Rep Oncol Med 2019 (60) | Adeno | NA | NA | Nivo, II | 7 months; imaging (previous PR) | Yes | Lung | Yes | 10+ months | Lung parenchyma with necrotic areas and reactive inflammatory changes. No tumor cells |
−, “negative” smoking history (never smokers); +, “positive” smoking history (current or former smokers). IHC, immunohistochemistry; PFS, progression-free survival; Adeno, adenocarcinoma; NOS, not otherwise specified; NA, not available; Nivo, nivolumab; Ipi, ipilimumab; Pembro, pembrolizumab; CRS, cytokine release syndrome; PR, partial response; T, primary lung tumor; GGO, ground-glass opacities.
“Time-to-pseudoPD” may be in addition variable, ranging from days since treatment initiation (typically with the abrupt onset of symptomatology) to months after ICB start (more often represented by a pure radiological finding). Several reported events of pseudoPD are characterized by relevant clinical worsening and this challenges the idea that a radiological progression may be deemed a potential pseudoPD only if accompanied by a stable or improved clinical status. In one case, the initial pseudo-progressive phase was accompanied by a decrease in the serum tumor marker CYFRA 21-1 (54), suggesting a potential role for circulating biomarkers, including cell-free DNA (cfDNA), in addressing pseudoPD and HPD (see Section “Critical considerations”) (61).
If virtually every anatomic site seems to potentially harbor pseudoPD foci, they tend to develop almost exclusively within already known lesions, making the appearance of a new lesion suspect for real progression. PET imaging, whose use could potentially help to determine the nature of progression (pseudoPD versus real PD) does not add particular information, as immune cells infiltrating tumor sites are metabolically active and their recruitment may mimic real progression even at functional imaging.
Remarkably, the most relevant features of pseudoPD are represented by pathologic ones, with an enrichment in immune cells (especially T lymphocytes) to the detriment of tumor ones. In this sense, the concept of re-biopsy, introduced in the field of precision medicine in NSCLC as a tool to address molecular treatment, can be favorably translated in the immunotherapy domain as an element of differential diagnosis between real and pseudo progression.
PseudoPD management relies on symptomatic therapy in cases that require it; several patients reported in Table 2 received indeed steroid therapy, mainly in order to reduce mass effects, and pleuro- or pericardio-centesis were performed in the case of serous effusions. Albeit patients experiencing pseudoPD may have a worse prognosis compared to the ones who obtain a typical response (37), maintaining ICB when pseudoPD is clinically suspected may nevertheless engender positive survival outcomes, as beyond-progression strategies are advantageous (22,62).
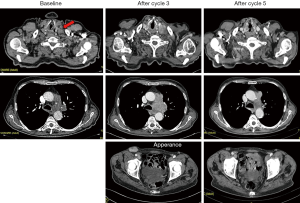
Figure 2 depicts the unpublished history of a patient experiencing pseudoPD while undergoing pembrolizumab for a highly expressing PD-L1 (≥50%) NSCLC.
Critical considerations
ICB are a pillar of NSCLC cancer therapy and their role appears in progressive widening, as beside advanced disease, new disease setting such as the locally-advanced and the localized ones are respectively already treated and under clinical evaluation (63,64). In this regard, the awareness concerning HPD and pseudoPD should be therefore even more reinforced.
Putting evidence in clinical context, as reported pseudoPD and HPD patterns in NSCLC have been described only in the advanced setting and almost only when anti-PD-1/PD-L1 agents were administered as monotherapy (more rarely, in combination with anti-CTLA-4 drugs). No evidence is thus far available regarding the potential biologic differences between metastatic diseases and less extended ones, that could account for a lower predisposition to HPD and pseudoPD. The rapidly evolving scenario of NSCLC treatment is defining combinatorial therapies involving ICB as the first-line treatment of advanced disease, involving anti-PD-1/PD-L1 combined either with chemotherapy (and anti-angiogenic compounds) or CTLA-4 agents (65). Again without concrete proofs, due to their mechanism of action, it may be speculated that chemotherapy would downsize the possibility of both HPD and pseudoPD, whereas anti-CTLA-4 agents, synergizing with anti-PD-1/PD-L1 ones in fostering immune system, may maintain some degree of risk. In this regard, while in clinical trials testing first-line pembrolizumab or atezolizumab/bevacizumab in combination with platinum-based chemotherapy [Keynote-189, Keynote-407, IMpower150 (66-68)], a clear and early separation of survival curves is observed, in CheckMate 227 comparing first-line nivolumab and ipilimumab vs platinum based chemotherapy (69), PFS curves crosses between three and six months and the progression rate is higher in the double immune checkpoint blockade arm (15.8% vs. 11.9%). These data could suggest a decreased risk of HPD with chemotherapy-immunotherapy combinations and a potential high rate of HPD with PD-1/PD-L1 inhibitors combined with anti-CTLA-4 ones. In CheckMate 227, HPD could also explain the lower access to subsequent treatments in patients discontinuing double immune checkpoint blockade for reasons other than toxicity: 34.4% versus 49.2% in the chemotherapy arm. With regard to pseduoPD, its occurrence has not been reported in published clinical trials evaluating the mentioned ICB combinations for the treatment of untreated NSCLC patients (66-69).
Nevertheless, ICB monotherapy will still be an option in the first-line treatment of PD-L1 ≥50% cases and for patients who cannot afford a combination regimen as first-line therapy for clinical reasons, thus reinforcing the attention pseudoPD and HPD will deserve even in the next future.
Interestingly, pseudoPD may sometimes radiologically mimic HPD, in fact 10.7% of NSCLC patients with HPD at first CT-scan subsequently experienced tumor shrinkage and prolonged clinical benefit and were classified as pseudoprogressors (20). In this regard, it will be crucial to distinguish between HPD and pseudoPD as early as possible, even before re-imaging. In this regard, a recent study showed that genome instability number in cfDNA rises rapidly with HPD but falls with pseudoPD when measured at 3–6 weeks after immunotherapy start (61). Finally, it could be difficult to differentiate the early disease flare which may occur after the end of an EGFR-TKI treatment (70), from HPD and pseudoPD, especially if ICB is used immediately after EGFR-TKI therapy discontinuation. However, in most of the studies few EGFR mutated patients were included and HPD was not related to the previous treatment type.
Finally, if pseudoPD does not harbor an intrinsic detrimental effect (i.e., patients experiencing this pattern of response still benefit from immunotherapy), HPD represent an element of real unmet clinical need. In this case indeed ICB generate harm, and efforts should be addressed towards the prediction of patients more likely to experience HPD. Three major elements may be suggested aiming to reduce HPD impact: (I) provide an common definition of HPD supported also by scientific societies and regulatory agencies, (II) continuing in studying cellular mechanisms involved in HPD, in order to (III) identify common clinical, pathological, radiological and immunological predictors of HPD in order to avoid single agent immune checkpoint blockade in these patients in favor of experimental immunotherapies or combinatorial treatment strategies.
Concluding remarks
The astonishing benefit generated by immunotherapy in NSCLC can be resumed by the long-term survival outcomes observed even in the advanced setting, among which a non-negligible quote of pretreated patients (15–20%) can be deemed potentially cured (3). These unprecedented results have not been obtained without a deal of drawbacks, such as HPD and pseudoPD. The understanding, recognition and management of the two response patterns is crucial in the perspective of a progressing improvement in survival rates, thanks to the incorporation of ICB in settings other than the advanced one and with combinatorial therapy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors Alfredo Addeo and Giuseppe Banna for the series “Non-Small Cell Lung Cancer (NSCLC)” published in Precision Cancer Medicine. The article has undergone external peer review.
Conflicts of Interest: The series “Non-Small Cell Lung Cancer (NSCLC)” was commissioned by the editorial office without any funding or sponsorship. F Facchinetti has no conflicts of interest to declare. G Lo Russo declares travel accommodation and consultancies from Roche, BMS, MSD, Astra-Zeneca. M Tiseo declares advisory boards and speakers’ fee for Astra-Zeneca, Pfizer, Eli-Lilly, BMS, Novartis, Roche, MSD, Boehringer Ingelheim, Otsuka, Takeda, Pierre Fabre. Research grants: Astra-Zeneca, Boehringer Ingelheim. MC Garassino declares personal financial interests with the following organizations: AstraZeneca, MSD International GmbH, BMS, Boehringer Ingelheim Italia S.p.A, Celgene, Eli Lilly, Ignyta, Incyte, Inivata, MedImmune, Novartis, Pfizer, Roche and Takeda; she also declares institutional financial interests with the following organizations: Eli Lilly, MSD, Pfizer (MISP), AstraZeneca, MSD International GmbH, BMS, Boehringer Ingelheim Italia S.p.A, Celgene, Ignyta, Incyte, Inivata, MedImmune, Novartis, Pfizer, Roche, Takeda, Tiziana and Foundation Medicine; she has received research funding from the following organizations: AIRC, AIFA, Italian Moh and TRANSCAN. R Ferrara declares having attended advisory board of MSD and having received travel grants from Pfizer. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv119-iv142. [Crossref] [PubMed]
- Prelaj A, Tay R, Ferrara R, et al. Predictive biomarkers of response for immune checkpoint inhibitors in non-small-cell lung cancer. Eur J Cancer 2019;106:144-59. [Crossref] [PubMed]
- Rebuzzi SE, Leonetti A, Tiseo M, et al. Advances in the prediction of long-term effectiveness of immune checkpoint blockers for non-small-cell lung cancer. Immunotherapy 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Ferrara R, Mezquita L, Texier M, et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 Inhibitors or with single-agent chemotherapy. JAMA Oncol 2018;4:1543-52. [Crossref] [PubMed]
- Lo Russo G, Moro M, Sommariva M, et al. Antibody-Fc/FcR interaction on macrophages as a mechanism for hyperprogressive disease in non-small cell lung cancer subsequent to PD-1/PD-L1 blockade. Clin Cancer Res 2019;25:989-99. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Garassino MC, Cho BC, Kim JH, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol 2018;19:521-36. [Crossref] [PubMed]
- Champiat S, Dercle L, Ammari S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res 2017;23:1920-8. [Crossref] [PubMed]
- Kato S, Goodman A, Walavalkar V, et al. Hyperprogressors after immunotherapy: Analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res 2017;23:4242-50. [Crossref] [PubMed]
- Saâda-Bouzid E, Defaucheux C, Karabajakian A, et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol 2017;28:1605-11. [Crossref] [PubMed]
- Singavi AK, Menon S, Kilari D. Predictive biomarkers for hyper-progression (HP) in response to immune checkpoint inhibitors (ICI) - analysis of somatic alterations (SAs). Ann Oncol 2017;28:1140PD.
- Matos I, Martin-Liberal J, Hierro C, et al. Incidence and clinical implications of a new definition of hyperprogression (HPD) with immune checkpoint inhibitors (ICIs) in patients treated in phase 1 (Ph1) trials. J Clin Oncol 2018;36:abstr 3032.
- Kim CG, Kim KH, Pyo KH, et al. Hyperprogressive disease during PD-1/PD-L1 blockade in patients with non-small-cell lung cancer. Ann Oncol 2019; [Epub ahead of print]. [PubMed]
- Sasaki A, Nakamura Y, Mishima S, et al. Predictive factors for hyperprogressive disease during nivolumab as anti-PD1 treatment in patients with advanced gastric cancer. Gastric Cancer 2019;22:793-802. [Crossref] [PubMed]
- Kim Y, Kim CH, Kim HS, et al. Hyperprogression after immunotherapy: Clinical implication and genomic alterations in advanced non-small cell lung cancer patients (NSCLC). J Clin Oncol 2018;36:abstr 9075.
- Kanjanapan Y, Day D, Wang L, et al. Hyperprogressive disease in early-phase immunotherapy trials: Clinical predictors and association with immune-related toxicities. Cancer 2019;125:1341-9. [Crossref] [PubMed]
- Kamada T, Togashi Y, Tay C, et al. PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc Natl Acad Sci U S A 2019;116:9999-10008. [PubMed]
- Xiong D, Wang Y, Singavi AK, et al. Immunogenomic landscape contributes to hyperprogressive disease after anti-PD-1 immunotherapy for cancer. iScience 2018;9:258-77.
- Ratner L, Waldmann TA, Janakiram M, et al. Rapid progression of adult T-cell leukemia-lymphoma after PD-1 inhibitor therapy. N Engl J Med 2018;378:1947-8. [Crossref] [PubMed]
- Ferrara R, Caramella C, Besse B. Hyperprogression-Immunotherapy-related phenomenon vs intrinsic natural history of cancer-In reply. JAMA Oncol 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Kim Y, Kim CH, Lee HY, et al. Comprehensive clinical and genetic characterization of hyperprogression based on volumetry in advanced non-small cell lung cancer treated with immune checkpoint inhibitor. J Thorac Oncol 2019; [Epub ahead of print]. [PubMed]
- Gandara DR, von Pawel J, Mazieres J, et al. Atezolizumab treatment beyond progression in advanced NSCLC: Results from the randomized, phase III OAK study. J Thorac Oncol 2018;13:1906-18. [Crossref] [PubMed]
- Understanding hyperprogression in cancer. Cancer Discov 2019;9:821. [Crossref] [PubMed]
- Champiat S, Ferrara R, Massard C, et al. Hyperprogressive disease: Recognizing a novel pattern to improve patient management. Nat Rev Clin Oncol 2018;15:748-62. [Crossref] [PubMed]
- Wartewig T, Kurgyis Z, Keppler S, et al. PD-1 is a haploinsufficient suppressor of T cell lymphomagenesis. Nature 2017;552:121-5. [Crossref] [PubMed]
- Ludin A, Zon LI. Cancer immunotherapy: The dark side of PD-1 receptor inhibition. Nature 2017;552:41-2. [Crossref] [PubMed]
- Mezquita L, Auclin E, Ferrara R, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol 2018;4:351-7. [Crossref] [PubMed]
- Delgado-López PD, Riñones-Mena E, Corrales-García EM. Treatment-related changes in glioblastoma: a review on the controversies in response assessment criteria and the concepts of true progression, pseudoprogression, pseudoresponse and radionecrosis. Clin. Transl. Oncol 2018;20:939-53. [Crossref] [PubMed]
- Ribas A, Chmielowski B, Glaspy JA. Do we need a different set of response assessment criteria for tumor immunotherapy? Clin Cancer Res 2009;15:7116-8. [Crossref] [PubMed]
- Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin Cancer Res 2009;15:7412-20. [Crossref] [PubMed]
- Nishino M, Giobbie-Hurder A, Gargano M, et al. Developing a common language for tumor response to immunotherapy: Immune-related response criteria using unidimensional measurements. Clin Cancer Res 2013;19:3936-43. [Crossref] [PubMed]
- Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017;18:e143-52. [Crossref] [PubMed]
- Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): A phase 2, single-arm trial. Lancet Oncol 2015;16:257-65. [Crossref] [PubMed]
- Nishino M, Ramaiya NH, Chambers ES, et al. Immune-related response assessment during PD-1 inhibitor therapy in advanced non-small-cell lung cancer patients. J Immunother Cancer 2016;4:84. [Crossref] [PubMed]
- Katz SI, Hammer M, Bagley SJ, et al. Radiologic pseudoprogression during anti-PD-1 therapy for advanced non-small cell lung cancer. J Thorac Oncol 2018;13:978-86. [Crossref] [PubMed]
- Kazandjian D, Keegan P, Suzman DL, et al. Characterization of outcomes in patients with metastatic non-small cell lung cancer treated with programmed cell death protein 1 inhibitors past RECIST version 1.1-defined disease progression in clinical trials. Semin Oncol 2017;44:3-7. [Crossref] [PubMed]
- Fujimoto D, Yoshioka H, Kataoka Y, et al. Pseudoprogression in previously treated patients with non-small cell lung cancer who received nivolumab monotherapy. J Thorac Oncol 2019;14:468-74. [Crossref] [PubMed]
- Ferrara R, Caramella C, Besse B, et al. Pseudoprogression in non-small cell lung cancer upon immunotherapy: Few drops in the ocean? J Thorac Oncol 2019;14:328-31. [Crossref] [PubMed]
- Hendriks LEL, Henon C, Auclin E, et al. Outcome of patients with non-small cell lung cancer and brain metastases treated with checkpoint inhibitors. J Thorac Oncol 2019;14:1244-54. [Crossref] [PubMed]
- Doherty MK, Jao K, Shepherd FA, et al. Central nervous system pseudoprogression in a patient treated with PD-1 checkpoint inhibitor. J Thorac Oncol 2015;10:e100-1. [Crossref] [PubMed]
- Ito K, Hataji O, Katsuta K, et al. “Pseudoprogression” of pulmonary pleomorphic carcinoma during nivolumab therapy. J Thorac Oncol 2016;11:e117-9. [Crossref] [PubMed]
- Kolla BC, Patel MR. Recurrent pleural effusions and cardiac tamponade as possible manifestations of pseudoprogression associated with nivolumab therapy- a report of two cases. J Immunother Cancer 2016;4:80. [Crossref] [PubMed]
- Tanizaki J, Hayashi H, Kimura M, et al. Report of two cases of pseudoprogression in patients with non-small cell lung cancer treated with nivolumab-including histological analysis of one case after tumor regression. Lung Cancer 2016;102:44-8. [Crossref] [PubMed]
- Curioni-Fontecedro A, Ickenberg C, Franzen D, et al. Diffuse pseudoprogression in a patient with metastatic non-small-cell lung cancer treated with Nivolumab. Ann Oncol 2017;28:2040-1. [Crossref] [PubMed]
- Izumida T, Kawagishi Y, Tsuji H. Pseudoprogression in lung adenocarcinoma during treatment with nivolumab. BMJ Case Rep 2017; [Crossref] [PubMed]
- Kato R, Hayashi H, Tanizaki J, et al. Peritumoural ground-glass opacity associated with tumour pseudoprogression in a patient with non-small cell lung cancer treated with nivolumab. ESMO Open 2017;2:e000145. [Crossref] [PubMed]
- Sarfaty M, Moore A, Dudnik E, et al. Not only for melanoma. Subcutaneous pseudoprogression in lung squamous-cell carcinoma treated with nivolumab. Medicine (Baltimore) 2017;96:e5951. [Crossref] [PubMed]
- Nishino M, Dahlberg SE, Adeni AE, et al. Tumor response dynamics of advanced non-small cell lung cancer patients treated with PD-1 inhibitors: Imaging markers for treatment outcome. Clin Cancer Res 2017;23:5737-44. [Crossref] [PubMed]
- Hochmair MJ, Schwab S, Burghuber OC, et al. Symptomatic pseudo-progression followed by significant treatment response in two lung cancer patients treated with immunotherapy. Lung Cancer 2017;113:4-6. [Crossref] [PubMed]
- Melian M, Lorente D, Aparici F, et al. Lung brain metastasis pseudoprogression after nivolumab and ipilimumab combination treatment. Thorac Cancer 2018;9:1770-3. [Crossref] [PubMed]
- Rocha P, Hardy-Werbin M, Naranjo D, et al. CD103+CD8+ lymphocytes characterize the immune infiltration in a case with pseudoprogression in squamous NSCLC. J Thorac Oncol 2018;13:e193-6. [Crossref] [PubMed]
- Swami U, Smith M, Zhang J, et al. Central nervous system pseudoprogression with nivolumab in a patient with squamous cell lung cancer followed by prolonged response. J Thorac Oncol 2018;13:e183-4. [Crossref] [PubMed]
- Yamaura T, Suzuki H. Pseudoprogression and rapid response to pembrolizumab in a patient with advanced lung adenocarcinoma with loss of epidermal growth factor receptor gene mutation after tyrosine kinase inhibitor therapy. J Thorac Oncol 2018;13:e209-10. [Crossref] [PubMed]
- Yoshimura A, Takumi C, Tsuji T, et al. Pulmonary pleomorphic carcinoma with pseudoprogression during nivolumab therapy and the usefulness of tumor markers: A case report. Clin Case Rep 2018;6:1338-41. [Crossref] [PubMed]
- Laurie SA, Banerji S, Blais N, et al. Canadian consensus: oligoprogressive, pseudoprogressive, and oligometastatic non-small-cell lung cancer. Curr Oncol 2019;26:e81-93. [Crossref] [PubMed]
- Kim HK, Baek SW, Jeong Y, et al. Pseudoprogression presenting as intestinal perforation in non-small cell lung cancer treated with anti-PD-1: A case report. Mol Clin Oncol. 2019;11:132-4. [PubMed]
- Asai M, Kato Y, Kawai S, et al. Management of cardiac tamponade during nivolumab of lung cancer with intrapericardial bleomycin: case report. Immunotherapy 2019;11:467-72. [Crossref] [PubMed]
- Kogure Y, Ishii Y, Oki M. Cytokine release syndrome with pseudoprogression in a patient with advanced non-small cell lung cancer treated with pembrolizumab. J Thorac Oncol 2019;14:e55-7. [Crossref] [PubMed]
- Masuhiro K, Shiroyama T, Nagatomo I, et al. Unique case of pseudoprogression manifesting as lung cavitation after pembrolizumab treatment. J Thorac Oncol 2019;14:e108-9. [Crossref] [PubMed]
- Suyanto S, Yeo D, Khan S. A rare delayed atypical pseudoprogression in nivolumab-treated non-small-cell lung cancer. Case Rep Oncol Med 2019;2019:8356148. [Crossref] [PubMed]
- Jensen TJ, Goodman AM, Kato S, et al. Genome-wide sequencing of cell-free DNA identifies copy-number alterations that can be used for monitoring response to immunotherapy in cancer patients. Mol Cancer Ther 2019;18:448-58. [Crossref] [PubMed]
- Ricciuti B, Genova C, Bassanelli M, et al. Safety and efficacy of nivolumab in patients with advanced non-small-cell lung cancer treated beyond progression. Clin Lung Cancer 2019;20:178-185.e2. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018;379:2342-50. [Crossref] [PubMed]
- Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med 2018;378:1976-86. [Crossref] [PubMed]
- Proto C, Ferrara R, Signorelli D, et al. Choosing wisely first line immunotherapy in non-small cell lung cancer (NSCLC): What to add and what to leave out. Cancer Treat Rev 2019;75:39-51. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 2018;378:2093-104. [Crossref] [PubMed]
- Akamatsu H, Ono A, Shukuya T, et al. Disease flare after gefitinib discontinuation. Respir Investig 2015;53:68-72. [Crossref] [PubMed]
Cite this article as: Facchinetti F, Lo Russo G, Tiseo M, Garassino MC, Ferrara R. How to recognize and manage hyper-progression and pseudo-progression during immune checkpoint blockade in non-small cell lung cancer. Precis Cancer Med 2019;2:35.

