
Implementation of functional precision medicine for anaplastic lymphoma kinase-rearranged non-small lung cancer
Introduction
Non-small cell lung cancer (NSCLC) accounts for about 85% of all lung cancer cases and is often detected at an advanced stage with poor prognosis. Systemic therapy consisted of cytotoxic chemotherapy with only a minor improvement in the median overall survival (OS) of less than 1 year from diagnosis (1). However, the discovery of specific genetic alterations in subpopulations of NSCLC patients and the subsequent development of targeted therapy have dramatically changed the outlook of NSCLC patients (2). Besides the more frequent epidermal growth factor receptor (EGFR) mutations, rearrangements of anaplastic lymphoma kinase (ALK) gene is present in 3–7% of NSCLCs with increased prevalence in adenocarcinoma histology, never-smokers or light-smokers and lower age compared to other lung cancer populations (3-5). ALK is a tyrosine kinase of the insulin receptor family with a physiological function in normal cell proliferation and neurogenesis (6). Following the detection of ALK rearrangement in lymphomas, oncogenic echinoderm microtubule associated protein like 4 (EML4)-ALK rearrangements were reported in NSCLC in 2007 (3). The downstream activity of ALK activation in cancer results in an increased cell proliferation and metabolism, cytoskeleton remodeling, migration and increased survival (7). Pleiotrophin and midkine are known ligands for this receptor (8). Patients are never-smokers or light smokers (less than 10 pack-years). Tumors generally tend to be more centrally located, and patients often present with advanced disease. Cerebral and hepatic metastases are common, as well as pleural and pericardial effusions which seems to affirm the inherent aggressive nature of this cancer (9,10).
The MET proto-oncogene tyrosine kinase inhibitor (TKI) crizotinib was found to constitute an inhibitor of rearranged ALK and the phase I trial PROFILE 1001 led to the approval of crizotinib by the US Food and Drug Administration (FDA) for patients with advanced ALK-rearranged NSCLC in 2011 (11). Comparison of ALK inhibitors to chemotherapy showed that crizotinib and the second-line ALK inhibitors ceritinib and alectinib prolong the progression free survival (PFS) and reveal a significantly better overall response rate (ORR) compared to chemotherapy in the first line as well as second line treatment regimens (12). Furthermore, the intracranial response rate was better with ALK inhibitors compared to chemotherapy. Overall ALK inhibitors are safe and effective treatment option in ALK-positive NSCLC. Currently, four first- and second-generation ALK inhibitors, crizotinib, ceritinib, alectinib, and brigatinib, as well as the third-line inhibitor lorlatinib are approved for clinical practice and many more are under development (13).
ALK rearrangement
The ALK gene was initially discovered in 1994 in anaplastic large-cell lymphoma and in 2007, Soda et al. identified the first ALK rearrangement in NSCLC, occurring between this gene and the EML4 implying a large inversion or translocation (3). The ALK gene belongs to the insulin receptor superfamily, and encodes for a transmembrane tyrosine kinase receptor, comprising an extracellular domain, a transmembrane segment, and a cytoplasmic receptor kinase segment. The EML4-ALK translocation leads to a driver mutation with high oncogenic activity located in the cytoplasm (Figure 1). Whereas the breakpoint of ALK is at the exon 20, many fusion variants are generated by fusion with different breakpoints in the EML4 exons 2, 6, 13, 14, 15, 18 and 20, differing in frequencies from V1 (54.5%), V2 (10%), V3a/V3b (34%), to V5a (1.5%) (14-16). The association between outcomes to specific EML4-ALK variants as potential predictive markers for response is rarely investigated so far (17). A study examined 67 stage IV lung cancer patients with EML4-ALK fusion variants 1, 2, and 3a/3b and concluded that the V3 (3a/3b) variant is associated with more metastatic sites at diagnosis, earlier failure after treatment with first or second line ALK inhibitors, platinum-based chemotherapy, and cerebral radiotherapy, resulting in an inferior OS (16,18). Dimerization of the ALK kinase domains triggers canonical pathways such as MAPK, PI3K/mTOR, JAK-STAT, SHH among others. The fusion protein is under control of the EML4 gene promotor which results in ALK overexpression and constitutive tyrosine kinase activity (19). Furthermore, more than 19 different ALK fusion partners have been discovered in NSCLC, including EML4, KIF5B, KLC1, and TPR (20). The first drug resistance point mutations identified were C1156Y and L1196M. Subsequently, several other point mutations conferring drug resistance have been identified, including: G1269A, F1174L, 1151Tins, L1152R, S1206Y, I1171T, G1202, D1203N, and V1180L. As a screening test, ALK immunohistochemistry (IHC), which can be performed short term at low cost, requires less effort and expertise than fluorescence in situ hybridization (FISH) which is in general use (21,22). In addition, next-generation sequencing (NGS) identifies ALK fusion partner genes, ALK mutations, and non-target alterations (23). The sensitivity of a particular NGS assay is affected by confounding factors such as tumor cell purity (dilution of tumor signal with DNA from normal cells) and presence of multiple clonal tumor cell populations within a given sample (intratumor heterogeneity or clonal diversity). However, whole transcriptome sequencing may not be cost-effective for thousands of patients and longer turnaround times are not conducive for clinical decision-making. For instance, the genetic makeup of metastatic tumors has been shown to differ substantially from the primary tumor (24). Additionally, tumors frequently develop resistance to targeted therapies, due to both acquired de novo resistance mechanisms and Darwinian selection of pre-existing resistant subclones. The latter mechanism in particular can lead to rapid development of resistance and disease progression (25,26).
Treatment of ALK-rearranged NSCLC
Crizotinib was the first ALK TKI shown to be superior to chemotherapy in randomized phase III trials revealing significant improvement in ORR with targeted therapy (65% vs. 20%) (27,28). Thus, crizotinib was established as a new standard of care in second- and first-line therapy, respectively, which, furthermore, improves quality of life compared with chemotherapy. In the second line setting, crizotinib showed an ORR of 65% and 4 months of PFS-benefit in comparison with docetaxel or pemetrexed. Inevitably and like EGFR inhibition with EGFR TKIs, resistance to ALK inhibition develops in an average of 1 year (29). Only 30% of cases of acquired crizotinib resistance in patients with ALK-rearranged NSCLC stem from various secondary mutations of ALK, with the remaining 70% of such cases being due to other mechanisms and the vast majority of new or progressing lesions develop intracranially (30,31). After the failure of crizotinib, the second-line inhibitors ceritinib and alectinib have shown clinical responses. Additionally, the newer ALK TKIs generally provide more pronounced intracranial activity than crizotinib. The FDA granted accelerated approval of ceritinib in April 2014, for patients who progressed while receiving crizotinib. Alectinib received a similar approval for the same population in December 2015 followed by brigatinib in April 2017 (32).
Resistance to ALK inhibitors
Mechanisms of resistance to ALK TKIs are classified as either ALK-dependent “on-target” mechanism including secondary ALK mutations and amplifications or ALK-independent “off-target” mechanisms involving alternative signaling pathways and lineage transformations (33,34). In crizotinib resistance, secondary mutations occur in 20–30%, but for patients resistant to next-generation ALK-inhibitors, the frequency of ALK secondary resistance mutations increases to 50–70%. The occurrence of the resistant G1202R mutation is common and represents 21%, 29% and 43% of patients in cases resistant to ceritinib, alectinib and brigatinib, respectively (35). Lorlatinib, the third-generation ALK inhibitor has been shown to overcome resistance to this mutation (36). Alternatively, ALK-independent mechanisms comprise alterations in EGFR, KRAS, BRAF, MET, HER2 and KIT (37). Consequently, this prompted the development of newer generation ALK TKIs to overcome these resistance patterns, and these drugs include ceritinib, alectinib, brigatinib, ensartinib and lorlatinib. Brigatinib has demonstrated a broad spectrum of preclinical activity against crizotinib-resistant ALK mutant NSCLC (38). Brigatinib acts as a multi-kinase inhibitor with a broad-spectrum activity against ALK, ROS1, FLT3, mutant variants of FLT3, IGFR-1R and T790M-mutant EGFR. Brigatinib displayed superior activity compared to crizotinib, ceritinib, and alectinib, against all 17 secondary ALK mutations, including C1156Y, I1171S/T, V1180L, L1196M, L1152R/P, E1210K, G1269A and the most refractory G1202R mutation (39). G1202R the only mutation so far associated with clinical resistance to all three previously approved ALK inhibitors.
Lorlatinib is the latest addition to the four targeted drugs currently available in the clinic via regular approval (5). Lorlatinib was specifically developed to cross the blood-brain barrier and to retain potency to acquired resistant mutations, including the ALK G1202R mutation. The frequency of this mutation increases significantly after treatment with second-generation agents (35). The presence of ALK resistance mutations is highly predictive for sensitivity to lorlatinib, whereas those cell lines without ALK mutations are resistant. Lorlatinib showed marked overall and intracranial activity both in treatment-naive patients and in those who had progressed on crizotinib, second-generation ALK inhibitors, or after up to three previous ALK inhibitors (40). Based on phase I and preliminary phase II data, lorlatinib received accelerated approval by the FDA in November 2018 for patients whose disease progressed on crizotinib or at least one other ALK inhibitor (alectinib or ceritinib). The ORR was 48%, with a complete response in 4% of patients and the estimated median duration of response 12.5 months. The intracranial ORR in 89 patients with measurable central nervous system (CNS) lesions was 60%, with a complete response in 21% and the estimated median duration of intracranial response was 19.5 months (40). A phase III study of lorlatinib vs crizotinib in first line treatment of patients with ALK-positive NSCLC is currently recruiting patients (NCT03052608).
A number of third-generation ALK inhibitors, such as entrectinib (RXDX-101) and ensartinib (X-396) is under trial and additional next-generation ALK inhibitors such as belizatinib (TSR-011), ASP3026, TPX-0005, F17752, CEP-37440, CEP- 28122, and GSK1838705A are under development (41). The new agents are expected to show enhanced anti-ALK activity, to improve the control of CNS disease, and to overcome or delay development of high-grade resistance mutations.
Sequence of the application of ALK inhibitors
The traditional approach to the treatment of patients with advanced-stage NSCLC harboring ALK rearrangements or EGFR mutations has been the sequential administration of therapies, in which patients first receive first-generation TKIs, which are eventually replaced by next-generation TKIs and/or chemotherapy upon disease progression, in a decision optionally guided by tumor molecular profiling (42). In the past few years, this strategy has been challenged by clinical evidence showing improved PFS, improved intracranial disease control and a generally favorable toxicity profile when next-generation EGFR and ALK TKIs are used in the first-line setting.
Experience with sequential treatment at our own center revealed that brigatinib as a second-line or later-line treatment in patients with ALK-rearranged NSCLC who developed resistance to crizotinib followed by various TKIs resulted in a disease control rate of 84.8% (43). Crizotinib was the single previous treatment for 15 (42.9%) of the patients, while crizotinib followed by ceritinib had been administered to 12 (34.3%) of the patients. Alectinib monotherapy had only been used for one patient, crizotinib followed by alectinib for two patients, crizotinib followed by ceritinib and alectinib for one patient, and ceritinib followed by alectinib for two patients (43). Seven of the 13 (53.8%) patients with brain metastases at baseline responded to brigatinib and the median PFS for the whole patient group was 9.9 months (range, 1–21 months). In all other subgroups (i.e., the various previous treatment combinations), all of the patients showed partial responses, with the exception of the two who had received ceritinib followed by alectinib which experienced disease progression under brigatinib.
The real problem is how to appropriately sequence therapy to obtain the most clinical benefit for the patients. The J-ALEX study introduced new insight into upfront treatment with second generation ALK inhibitors, demonstrating an improved PFS favoring alectinib (not estimable) over crizotinib (10.2 months) in the first line setting (HR 0.34). Brigatinib showed an ORR of 45% to 54% with a PFS of 9.2 to 12.9 months in the second-line setting (44). In conclusion, as distinct recommendations for subsequent therapies based on resistance mutation patterns are lacking, testing of these patterns will most likely not gain the same acceptance in clinical practice as for EGFR resistance mutation testing, at least not in the short term (43).
ALK inhibitors and precision medicine
The progress of genomic profiling and NGS enables patient-centered targeted therapy, now termed precision cancer medicine (45). In NSCLC ALK-positive patients, the knowledge about primary and, particularly, secondary resistance mutations were expected to facilitate the selection of the most optimal treatment sequence. The US National Cancer Institute (NCI) is developing a “Master Protocol” for the selection of the treatment of patients with ALK-rearranged advanced NSCLC, in which the different mutations will guide therapy and drug sequence (46). Such a protocol is defined as a comprehensive operating procedure aiming at evaluating multiple hypotheses of sub-studies which are commonly conducted on cohorts based on specific tumor types, histologic subtypes, and/or molecular markers (47).
However, alterations of ALK fusion proteins are complex and comprise a number of distinct fusion partners and variants as well as a range of mutations providing distinct chemosensitivities and mechanisms of resistance to inhibitors. In genome-guided therapy, the relationship of drug sensitivity to various alterations of the target in a variable cellular environment may be difficult to establish. Actually, genomic-based cancer precision medicine has so far been successfully applied only for small minority of patients with cancer for which matching drugs have been available (48,49). The situation in ALK rearranged NSCLC is unique insofar as a number of alterations linked to chemoresistance is known and, in contrast to other cancers and various genetic alterations, a large collection of efficacious drugs is available.
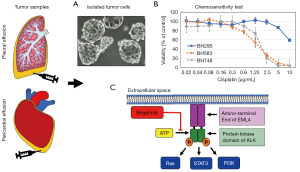
A more direct correlation between the characteristics of individual tumors and their chemosensitivity is investigated in the frame of the so-called functional precision medicine. In detail, tumor cells of patients are isolated and exposed in vitro to a range of appropriate drugs to test their sensitivity and guide clinical therapy in conjunction with genomic data. ALK-rearranged tumors progressing under therapy tend to grow aggressively and a major fraction of the patients show accumulation of pleural or pericardial fluid containing significant numbers of tumor cells. These cells can be easily collected by aspiration and immediately used for in vitro assays (50). In case of ALK-positive NSCLC, the whole range of inhibitors can be applied to select the most effective sequential drug matching the individual patient’s tumor. For these patients, rebiopsy or detection of ALK rearrangements in liquid biopsy is not necessary for selecting active agents (51).
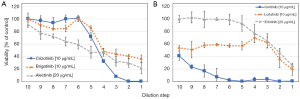
An example of such an ALK TKI sensitivity assay is shown in Figure 2. Tumor cells are collected from pleural or pericardial effusions and transferred to tissue culture. ALK-rearranged cells are often assembled as hollow spheres and partially attached to the culture flasks (Figure 2). Typical experiments showing the chemosensitivity of three NSCLC lines to cisplatin and ALK inhibitors are presented in Figures 1,2. Half maximal inhibitory concentrations (IC50) can be calculated from these dose-response curves and compared to the corresponding peak plasma concentrations to estimate the sensitivity of the tumor cells in the clinical situation. In particular, the ALK-rearranged tumor cells can be treated with all kinds of the respective TKIs and the likeliness of clinical response to a specific agent concluded. Additionally, the in vitro sensitivity data may be linked to the genome-wide alterations detected by NGS. Obstacles of this kind of tests may be the lack of sufficient tumor cells, questionable validity of the primary pleural tumor cells as representatives of the original tumor and tumor cell heterogeneity. Such assays are under development and need to be clinically validated in larger patient cohorts. However, these tests could identify the most active inhibitors within a short time frame in part of the patients allowing for short-term decision making.
Conclusions
Compared to chemotherapy, the survival of ALK-rearranged NSCLC patients had been markedly improved by treatment with inhibitors directed to the tyrosine kinase moiety of rearranged ALK. Treatment of ALK-rearranged NSCLC is a success story of targeted cancer therapy. The first successful inhibitor crizotinib is now being replaced by second-line drugs such as alectinib, ceritinib and brigatinib which show also improved activity as first-line agents. Most importantly, the newer ALK inhibitors are distinguished by high activity against brain metastases, a frequent site of secondary lesions in these patients. Development of resistance to all these drugs can be overcome in most patients by the third-line drug lorlatinib, although half of the patients which are refractory to treatment seem to have various mechanisms of resistance different from ALK mutations. Unfortunately, the handling of resistance mediated by ALK mutations is not as straightforward as in case of the progressive alterations of the EGFR in NSCLC. Currently, the optimal sequence of the administration of the ALK inhibitors or combinations thereof or with other therapeutics is not clear. Data on ALK rearrangement variants or mutations may be used to select the appropriate therapy. In the future, functional genomics studying the in vitro chemosensitivity of pleura-derived tumor cells may match individual tumors with effective drugs in suitable patients.
Acknowledgments
We thank Dr. T. Hohenheim for helpful support.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pcm.2019.05.03). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92-8. [Crossref] [PubMed]
- Pacheco JM, Gao D, Smith D, et al. Natural history and factors associated with overall survival in stage IV ALK rearranged non-small-cell lung cancer. J Thorac Oncol 2019;14:691-700. [Crossref] [PubMed]
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [Crossref] [PubMed]
- Ali R, Arshad J, Palacio S, et al. Brigatinib for ALK-positive metastatic non-small-cell lung cancer: design, development and place in therapy. Drug Des Devel Ther 2019;13:569-80. [Crossref] [PubMed]
- Chia PL, Mitchell P, Dobrovic A, et al. Prevalence and natural history of ALK positive non-small-cell lung cancer and the clinical impact of targeted therapy with ALK inhibitors. Clin Epidemiol 2014;6:423-32. [Crossref] [PubMed]
- Ryser CO, Diebold J, Gautschi O. Treatment of anaplastic lymphoma kinase-positive non-small cell lung cancer: update and perspectives. Curr Opin Oncol 2019;31:8-12. [PubMed]
- Palmirotta R, Quaresmini D, Lovero D, et al. ALK gene alterations in cancer: biological aspects and therapeutic implications. Pharmacogenomics 2017;18:277-92. [Crossref] [PubMed]
- Rosas G, Ruiz R, Araujo JM, et al. ALK rearrangements: Biology, detection and opportunities of therapy in non-small cell lung cancer. Crit Rev Oncol Hematol 2019;136:48-55. [Crossref] [PubMed]
- Doebele RC, Lu X, Sumey C, et al. Oncogene status predicts patterns of metastatic spread in treatment-naive non-small cell lung cancer. Cancer 2012;118:4502-11. [Crossref] [PubMed]
- Kang HJ, Lim HJ, Park JS, et al. Comparison of clinical characteristics between patients with ALK-positive and EGFR-positive lung adenocarcinoma. Respir Med 2014;108:388-94. [Crossref] [PubMed]
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small lung cancer. N Engl J Med 2010;363:1693-703. [Crossref] [PubMed]
- Khan M, Lin J, Liao G, et al. ALK Inhibitors in the Treatment of ALK Positive NSCLC. Front Oncol 2019;8:557. [Crossref] [PubMed]
- Rocco D, Battiloro C, Della Gravara L, et al. Safety and Tolerability of Anaplastic Lymphoma Kinase Inhibitors in Non-Small-Cell Lung Cancer. Drug Saf 2019;42:199-209. [Crossref] [PubMed]
- Wang R, Pan Y, Li C, et al. The use of quantitative real-time reverse transcriptase PCR for 5' and 3' portions of ALK transcripts to detect ALK rearrangements in lung cancers. Clin Cancer Res 2012;18:4725-32. [Crossref] [PubMed]
- Heuckmann JM, Balke-Want H, Malchers F, et al. Differential protein stability and ALK inhibitor sensitivity of EML4-ALK fusion variants. Clin Cancer Res 2012;18:4682-90. [Crossref] [PubMed]
- Sabari JK, Santini FC, Schram AM, et al. The activity, safety, and evolving role of brigatinib in patients with ALK-rearranged non-small cell lung cancers. Onco Targets Ther 2017;10:1983-92. [Crossref] [PubMed]
- Patel M, Malhotra J, Jabbour SK. Examining EML4-ALK variants in the clinical setting: the next frontier? J Thorac Dis 2018;10:S4104-7. [Crossref] [PubMed]
- Christopoulos P, Endris V, Bozorgmehr F, et al. EML4-ALK fusion variant V3 is a high-risk feature conferring accelerated metastatic spread, early treatment failure and worse overall survival in ALK+ non-small cell lung cancer. Int J Cancer 2018;142:2589-98. [Crossref] [PubMed]
- Hallberg B, Palmer RH. Mechanistic insight into ALK receptor tyrosine kinase in human cancer biology. Nat Rev Cancer 2013;13:685-700. [Crossref] [PubMed]
- Du X, Shao Y, Qin HF, et al. ALK-rearrangement in non-small-cell lung cancer (NSCLC). Thorac Cancer 2018;9:423-30. [Crossref] [PubMed]
- Savic S, Diebold J, Zimmermann AK, et al. Screening for ALK in non-small cell lung carcinomas: 5A4 and D5F3 antibodies perform equally well, but combined use with FISH is recommended. Lung Cancer 2015;89:104-9. [Crossref] [PubMed]
- Wynes MW, Sholl LM, Dietel M, et al. An international interpretation study using the ALK IHC antibody D5F3 and a sensitive detection kit demonstrates high concordance between ALK IHC and ALK FISH and between evaluators. J Thorac Oncol 2014;9:631-8. [Crossref] [PubMed]
- Chen HZ, Bonneville R, Roychowdhury S. Implementing precision cancer medicine in the genomic era. Semin Cancer Biol 2019;55:16-27. [Crossref] [PubMed]
- Turajlic S, Swanton C. Metastasis as an evolutionary process. Science 2016;352:169. [Crossref] [PubMed]
- Jonsson VD, Blakely CM, Lin L, et al. Novel computational method for predicting polytherapy switching strategies to overcome tumor heterogeneity and evolution. Sci Rep 2017;7:44206. [Crossref] [PubMed]
- McGranahan N, Swanton C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell 2017;168:613-28. [Crossref] [PubMed]
- Blackhall F, Kim DW, Besse B, et al. Patient-reported outcomes and quality of life in PROFILE 1007: a randomized trial of crizotinib compared with chemotherapy in previously treated patients with ALK-positive advanced non-small cell lung cancer. J Thorac Oncol 2014;9:1625-33. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Solomon BJ, Kim DW, Wu YL, et al. Final Overall Survival Analysis from a Study Comparing First-Line Crizotinib Versus Chemotherapy in ALK-Mutation-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:2251-8. [Crossref] [PubMed]
- Iwama E, Okamoto I, Harada T, et al. Development of anaplastic lymphoma kinase (ALK) inhibitors and molecular diagnosis in ALK rearrangement-positive lung cancer. Onco Targets Ther 2014;7:375-85. [PubMed]
- Blackhall F, Ross Camidge D, Shaw AT, et al. Final results of the large-scale multinational trial PROFILE 1005: efficacy and safety of crizotinib in previously treated patients with advanced/metastatic ALK-positive non-small-cell lung cancer. ESMO Open 2017;2:e000219. [Crossref] [PubMed]
- Spagnuolo A, Maione P, Gridelli C. Evolution in the treatment landscape of non-small cell lung cancer with ALK gene alterations: from the first- to third-generation of ALK inhibitors. Expert Opin Emerg Drugs 2018;23:231-41. [Crossref] [PubMed]
- Katayama R, Friboulet L, Koike S, et al. Two novel ALK mutations mediate acquired resistance to the next-generation ALK inhibitor alectinib. Clin Cancer Res 2014;20:5686-96. [Crossref] [PubMed]
- Toyokawa G, Seto T. Updated evidence on the mechanisms of resistance to ALK inhibitors and strategies to overcome such resistance: clinical and preclinical data. Oncol Res Treat 2015;38:291-8. [Crossref] [PubMed]
- Gainor JF, Dardaei L, Yoda S, et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov 2016;6:1118-33. [Crossref] [PubMed]
- Zou HY, Friboulet L, Kodack DP, et al. PF-06463922, an ALK/ROS1 inhibitor, overcomes resistance to first and second generation ALK inhibitors in preclinical models. Cancer Cell 2015;28:70-81. [Crossref] [PubMed]
- Lin JJ, Riely GJ, Shaw A. Targeting ALK: precision medicine takes on drug resistance. Cancer Discov 2017;7:137-55. [Crossref] [PubMed]
- Reckamp K, Lin HM, Huang J, et al. Comparative efficacy of brigatinib versus ceritinib and alectinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small cell lung cancer. Curr Med Res Opin 2019;35:569-76. [Crossref] [PubMed]
- Zhang S, Anjum R, Squillace R, et al. The potent ALK inhibitor brigatinib (AP26113) overcomes mechanisms of resistance to first- and second-generation ALK inhibitors in preclinical models. Clin Cancer Res 2016;22:5527-38. [Crossref] [PubMed]
- Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. The Lancet Oncology 2018;19:1654-67. [Crossref] [PubMed]
- Ziogas DC, Tsiara A, Tsironis G, et al. Treating ALK-positive non-small cell lung cancer. Ann Transl Med 2018;6:141. [Crossref] [PubMed]
- Recondo G, Facchinetti F, Olaussen KA, et al. Making the first move in EGFR-driven or ALK-driven NSCLC: first-generation or next-generation TKI? Nat Rev Clin Oncol 2018;15:694-708. [Crossref] [PubMed]
- Hochmair M, Weinlinger C, Schwab S, et al. Treatment of ALK-rearranged non-small-cell lung cancer with brigatinib as second or later lines: real-world observations from a single institution. Anti-Cancer Drugs 2019; [Epub ahead of print].
- Spencer SA, Riley AC, Matthew A, et al. Brigatinib: Novel ALK Inhibitor for Non-Small-Cell Lung Cancer. Ann Pharmacother 2019;53:621-6. [Crossref] [PubMed]
- Katayama R, Lovly CM, Shaw AT. Therapeutic targeting of anaplastic lymphoma kinase in lung cancer: a paradigm for precision cancer medicine. Clin Cancer Res 2015;21:2227-35. [Crossref] [PubMed]
- Cecchini M, Rubin EH, Blumenthal GM, et al. Challenges with Novel Clinical Trial Designs: Master Protocols. Clin Cancer Res 2019;25:2049-57. [Crossref] [PubMed]
- Hirakawa A, Asano J, Sato H, et al. Master protocol trials in oncology: Review and new trial designs. Contemp Clin Trials Commun 2018;12:1-8. [Crossref] [PubMed]
- Pauli C, Hopkins BD, Prandi D, et al. Personalized In Vitro and In Vivo Cancer Models to Guide Precision Medicine. Cancer Discov 2017;7:462-77. [Crossref] [PubMed]
- Letai A. Functional precision cancer medicine-moving beyond pure genomics. Nat Med 2017;23:1028-35. [Crossref] [PubMed]
- Hamilton G, Rath B. Applicability of tumor spheroids for in vitro chemosensitivity assays. Expert Opin Drug Metab Toxicol 2018; [Epub ahead of print]. [PubMed]
- Wang Y, Tian PW, Wang WY, et al. Noninvasive genotyping and monitoring of anaplastic lymphoma kinase (ALK) rearranged non-small cell lung cancer by capture-based next-generation sequencing. Oncotarget 2016;7:65208-17. [PubMed]
Cite this article as: Hamilton G, Rath B, Plangger A, Hochmair M. Implementation of functional precision medicine for anaplastic lymphoma kinase-rearranged non-small lung cancer. Precis Cancer Med 2019;2:19.

