How to improve the precision of closed chest sublobar resections
The standard surgical treatment for non-small cell lung cancer (NSCLC) is pulmonary lobectomy, regardless of the stage of the cancer (1). However, trials are currently underway to compare survival between sublobar resection (SLR) and lobectomy for early stage-NSCLC. The results of these studies are expected within the next three years but, nevertheless, there is a growing interest in SLRs in the surgical community to treat some benign lesions, some metastases that cannot be removed by mere wedge resection, ground-glass opacities (GGOs) and even some early stage NSCLC. Reasons for this renewed interest are multiple: trying to improve patients’ quality of life by better preserving respiratory function (2) and reducing the morbidity of major pulmonary resections. The morbidity of standard lobectomies is indeed 40% and mortality is more than 2% (3). In the 1990s, basing on the conclusions of the Lung Cancer Study Group, it was claimed that SLRs had a higher recurrence rate than lobectomies and had a lower survival rate (4). This had been confirmed by cohort studies (5). But until now, the question had not been asked whether these studies might not include a bias related to the fact that the surgical technique used in SLR did not always match oncological principles (6). In many studies, it is unclear whether SLR are true anatomical resections (wedges and segmentectomies are frequently mixed) and if an adequate lymph node dissection is performed. Comparative studies between anatomical segmentectomy associated to lymph node dissection and lobectomy from high-volume expert centers do not show any significant difference in survival (7-11).
In this hypothesis, segmentectomies become attractive, especially when they are performed by thoracoscopic surgery. It has indeed been shown by several studies, including one from our department (12), that post-operative morbidity was significantly less by thoracoscopy than by thoracotomy. Nevertheless, the morbidity of thoracoscopic segmentectomies remains high, ranging from 15% to 30%, well above the morbidity of alternative techniques such as radiotherapy or radiofrequency, whose complication rate is <2% and whose mortality is null (13). Part of the morbidity of thoracoscopic segmentectomies can be explained by technical issues such as confusion in vascular anatomy and inappropriate management of the intersegmental plane (ISP). This is why one of our challenges is to drastically improve accuracy. The avenues for improvement are presented in this article.
Optimal understanding of anatomy
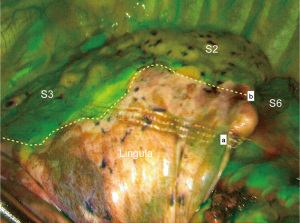
The bronchovascular anatomy of the pulmonary segments is complex and includes multiple variations (14-16). It can even be asserted there is no standard segmental anatomy. The concern related to the thoracoscopic approach is the difficulty to properly expose anatomical structures and the lack of global vision of the lung. The distribution and direction of arteries and veins is therefore often difficult to apprehend. For example, the opening of the fissure and extensive dissection of the vessels only allows them to be exposed over a limited length, so it is often problematic to state that a particular vessel is intended for a particular segment. This is why it is essential backing on mapping during the procedure but also beforehand to study anatomical variations in the preoperative period. This mapping is based on a three-dimensional (3D) reconstruction from an injected computed tomography (CT)-scanner. Most patients whose renal function is compatible with an injected CT can have a preoperative modelization.
Currently, several software programs allow navigation through the anatomy, layer by layer and superimposing elements, for example bronchi and arteries or bronchi and veins. In our department, we have found that when the surgeon has a cartography, it is used during surgery in more than 75% of cases, even if he/she has studied the reconstruction before the operation (Figures 1,2).
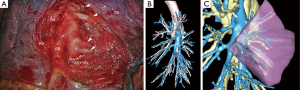
Several teams have demonstrated the study of preoperative computed tomography 3D reconstruction helps asserting the number and direction of arteries with good correlation. Oizumi et al. reported a correlation rate between 3D model and intraoperative findings of 98% (17). Eguchi et al. demonstrated in 14 consecutive patients the usefulness of manipulating the 3D CT navigation using a tablet interactively during the thoracoscopic procedure, helping them to perform the procedure safely and offering a valuable learning with regard to pulmonary vessel anatomy (18).
With the vascular pattern in mind the surgeon can perform a safer dissection of the pulmonary artery branches, especially when the fissure is fused and/or when lymph nodes are present. We use an online service that provides patient’s 3D modeling from medical DICOM images (Visible Patient™, Strasbourg, France). The images are anonymized and then uploaded on a secured web portal. A 3D reconstruction from these images is created according to the surgeon’s request. The 3D model can be used via a planning software and manipulated from any direction, either on a laptop, on a tablet or smartphone. All members of the surgical team have a direct access to the images on their own phone or tablet so that strategy and technical issues can be discussed within the group at any time. The software not only allows studying the main anatomical landmarks, in total or separately, but provides the following helpful functions: (I) display of virtual segmentectomy by clicking on a selected bronchus; (II) calculation of the volume of the resected segment; (III) calculation and simulation of a safety margin. This helps visualizing if the planned segmentectomy will have a safe margin, or if a more extended resection must be chosen for carcinological safety.
With growing surgical experience and with an extensive vascular dissection, it is possible to operate without reconstruction in some relatively simple segmentectomies. But in complex ones, 3D visualization is almost mandatory and will become part of the thoracic surgeon’s arsenal in a near future, just like any imaging system or equipment. Not only will the surgeon save time, but the patient will also gain in safety and overall the precision of the procedure will be improved. In this respect, it is interesting to note that in France, insurance companies are in the process of reimbursing these reconstructions.
Although 3D modeling is an insight, it is not yet the ideal tool and does not solve all orientation and comprehension problems during the procedure. The perfect system would be the use of augmented reality with overlaying on the screen of real and virtual anatomy. Unfortunately, it seems we have to wait several years for such a system. The technological challenge is indeed major because of the movements of the anatomical structures related to heartbeats and especially the fact that the models are made from a CT on a ventilated lung while the surgeon operates on a non-ventilated lung.
3D printing of the modelization can be an improvement as it can be manipulated manually and is more intuitive (19). The technology is still in its infancy and the costs are high, but progresses and cost cutting are expected.
Optimal approach
It is important to have a representation of the anatomical landmarks before and during the procedure. But this would be useless if the surgeon does not have an optimal exposure of anatomical structures. For thoracoscopic lobectomies, a “fissure first” approach is usually compared to a “fissure last” approach, which is theoretically faster and easier. For this reason, the latter technique—up to now—appeals most surgeons. However, for anatomical SLR, a large fissure opening is necessary to dissect segmental arteries and veins and detect the many anatomical variations (Figure 3). In the case of a fused fissure, the tunnel technique with opening of parenchyma by stapling along the arterial plane can help (20).
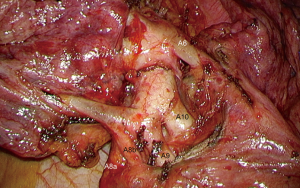
Appropriate exposure of anatomical structures, use of high-definition imaging combined or not with 3D display, use of dedicated instrumentation whose size is adapted to the diameter of bronchovascular elements, use of adequate hemostatic tools, are key elements to optimize the understanding and preservation of segmental anatomy as well as the quality of dissection (21).
Optimizing safety margins
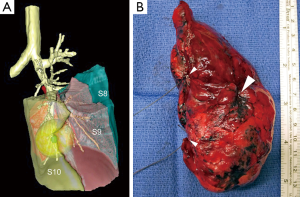
Compared to lobectomies, SLR comprise an increased risk of local recurrence as resection margins tend being closer to the tumor. Schuchert et al. have clearly demonstrated that segmentectomies outcome compares favorably with lobectomy for stage I NSCLC but that margin/tumor ratios of less than 1 are associated with a higher rate of recurrence (22). In this study, a margin/tumor diameter ratio exceeding 1, was associated with a significant reduction rates compared with ratios of less than 1 (25.0% versus 6.2%; P=0.0014). The rate of local recurrence was 7.7% and was correlated in most cases with a tumor margin less than 2 cm. Preoperative modeling can help making decision and plan the adequate SLR. On the software we are using, we have added an automatic calculation and representation of the safety margin. A similar concept has been developed by other teams (23) (Figure 4A). During the procedure, an intraoperative examination of the margins should be performed whenever the lesion is not very far from the staple line. As the pathologist cannot examine the entire staple line, we place 2 stitches on the area to be examined (Figure 4B). In our series, the margin was insufficient in 5 patients leading to extend the segmentectomy to the adjacent segment or to the lobe. It should be noticed that a borderline margin sometimes came as a surprise to the surgeon who thought section was widely distant from the tumor (24). Failing to examine intraoperative margins exposes the patient to a significant risk of local recurrence and may partly explain the poorer results of SLR in some multicentric series.
Performing an adequate lymph node dissection
Several studies suggest intersegmental lymph node clearance to play a major role in the results of segmentectomies. Wolf et al. have demonstrated that in early stage NSCLC, lobectomy was associated with longer overall and recurrence-free survival. SLRs were associated with an increased rate of local recurrence (16% versus 8%, P=0.1117). However, when lymph nodes were sampled during segmentectomy, local recurrence rate and overall and recurrence-free survival distributions were similar to those of lobectomy (25). Similar conclusions have been reported in a more recent paper by Cox et al. (26). In the beginning of our experience, we were clearing all intersegmental lymph nodes but asked for frozen section only when these looked suspicious. However, with a larger experience, we have been confused by benign looking intersegmental LN which were actually invaded so that we are now asking for a intraoperative pathological analysis even for a normal looking node (24).
Enhancing the precision of identification of the ISP
Inadequate determination and division of the ISP can determinate unsatisfactory oncological and surgical results, being the main cause of locoregional recurrence and impaired long-term survival following segmentectomy.
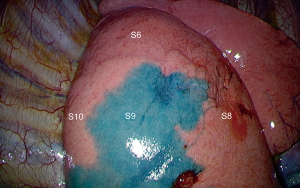
The conventional and most common maneuver used for delineation of the ISP consists of inflating the whole lung after occlusion of the target segmental bronchus inducing collapse of the segment to be resected and inflation of the remaining lung. However, because of the collateral ventilation through the pores of Kohn and because the inflated segments limits working space during thoracoscopic procedures, this method is unreliable. Some authors reported a method based on the use of near-infrared imaging system with indocyanine green (ICG) systemic injection after the division of the segmental vessels and bronchi by endostapler or clips, so that the fluorescence covered all structures except the isolated segment to be resected. The reported success rate ranges from 85% (27) to 100% (28). We are using systemic injection of ICG routinely in all our segmentectomy cases for 2 years and in our experience, the success rate is closer to 90% (Figure 5). It seems that poor vascularization of the parenchyma, as observed in patients presenting with chronic obstructive pulmonary disease (COPD), can be a cause of failure. According to Pischik and Kovalenko who had a 5% failure rate, COPD only reduces the duration of staining, but they observed bad results in patients with severe emphysema (29).
Endobronchial dye injection has also been reported (30). Sekine et al. demonstrated an accurate detection of ISP by maintaining the targeted colored segment when ICG injection into the associated bronchus followed by ligation of the segmental vein, was performed (31). Zhang et al. described a new technique consisting of methylene blue 0.1% (20 mL) injection into the bronchus of the target pulmonary segments through an intravenous needle after division of the vessels and bronchus of the segments to be resected (32).
Sato et al. described a virtual assisted pulmonary mapping (VAL-MAP), consisting of simultaneous marking of the pulmonary lesion to be removed and ISP on 3D images combining virtual bronchoscopy and endobronchial dye injection, achieving a success rate of 92.6% (33). Preoperative dye-injection can also be achieved via Electromagnetic Navigation Bronchoscopy (ENB) (30) (Figure 6).
Optimal method for dividing the ISP
Once identified, severing the ISP is another challenge. It was performed by electrocautery for a longtime. Because of the increasing number of anatomic SLR performed by thoracoscopy or video-assisted thoracic surgery (VATS), most surgeons are now using stapling. This method has been longtime criticized. Some authors reported that the stapling technique, may lead to plication of the parenchyma, impairing pulmonary re-expansion (34) while others demonstrated that stapling does not lead to less preserved function than electrocautery (35). The possible appearance of consolidation along the surgical stump has also been described has a complication after stapling the ISP. This finding could be related to the lesion of the intersegmental vein which has a role in the venous drainage of half of the preserved segment and which may be trapped in the staple line. According to these authors, the impaired venous drainage, could be the cause of chronic ischemia and subsequent parenchymal destruction (36).The clinical and radiologic support to these critics has not been investigated and has pushed us performing a CT study after segmentectomy. Our results are submitted to publication and under review. In summary, we have evaluated the presence of atelectasis, defined as an area of parenchymal consolidation larger than 5 mm in horizontal section view on CT scan, along the staple line, 3 to 4 months after the operation. We found only few atelectasis along the staple line. All of them were minor and the adjacent remaining segments were always well perfused with no sign of chronic ischemia. None of the atelectasis required surgery.
The development of the ISP by electrocautery, has been linked to prolonged air leak (PAL) (37) so that some authors described the use of various type of sealant and/or mesh. Matsumura et al. described better results with polyglycolic acid, an absorbable material that is able to closely adhere to irregular sections of the lung, effectively sealing air leakage and allowing a fast chest drain removal and excellent lung expansion (38). In a study from our department, there were only 8% patients who developed a PAL (39). These results are consistent with those reported in our previous series of 228 anatomic pulmonary segmentectomies (12) and lower than those reported by others who use to cut the ISP by electrocautery (40,41).
Miyasaka et al. compared electrocautery and the use of staplers for dividing the ISP during 49 segmentectomies (37). PALs were observed in 4 patients (8.2%). Three of these 4 patients underwent a chemical pleurodesis and one of them was reoperated for closing the air leak. None of these complications occurred in the stapler group (P=0.005). In a series of 102 hybrid VATS segmentectomy, electrocautery alone, i.e., without stapler, was used to divide the ISP and a fibrin sealant was applied along the raw surface of the remaining lung to prevent air leakage. PALs were observed in 4 patients (3.9%). Ohtsuka et al. compared 2 groups of patients having the ISP divided by electrocautery alone and electrocautery combined with stapling (42). The incidence of PAL was higher in the group electrocautery alone than in the group combination of electrocautery and staplers [14% (3/22) vs. 4% (1/25), P=0.025]. Thus, as mentioned by Miyasaka et al., dividing the ISP by electrocautery may result in more PALs (37).
Stapling the ISP is not yet the perfect method. Its handling is not that easy, especially in patients with small chest cavity. Loading thick tissues can be tedious, as their opening is limited, and disruption of the staple line can occur (43). Stapling the ISP seems to be a safe procedure. Our study showed that the rate of adjacent parenchyma atelectasis after intersegmental stapling during thoracoscopic anatomic segmentectomies is low (12.9%) and small in size with an average diameter of 8 mm. In view of these results, questions about the deleterious effect of stapling, on the viability of the adjacent parenchyma, do not seem justified. These findings do not prevent stapling from being carried out in the most precise and least traumatic way possible. This implies that all available technical means allowing for an accurate delineation of the ISP should be used, i.e., 3D modelization and intraoperative marking, whatever the method.
In total, it is possible to improve the precision of the SLRs. We have attempted summarizing the various means on Table 1. This is necessary to further reduce the morbidity of these procedures, which is already lower than that of lobectomies but still too high compared to non-surgical techniques. This requires (I) performing these procedures via a closed chest approach; (II) knowing as much as possible about segmental anatomy and studying mapping before and during the procedure; (III) performing an intraoperative examination on the intersegmental lymph nodes and on resection margins to expand resection if necessary; (IV) using a preoperative or intraoperative marking method to determine the ISP as accurately as possible; (V) stapling ISP, an imperfect method but that currently represents the least bad compromise between accuracy and safety.
Table 1
| To be optimized | Mean | Benefit | Limitation | Expected progress |
|---|---|---|---|---|
| Anatomy understanding | 3D modelization | Precise mapping | Cost | Augmented reality? |
| Time consuming | ||||
| Exposure | Clear display of anatomical structures | Time consuming | ||
| Can be difficult if fused fissure | ||||
| Determination of safety margins (with IOE) | 3D modelization | Preoperative determination of segments to be resected | Cost | |
| Time consuming | ||||
| Intraoperative examination | Allows immediate extension if invasion | Time consuming | On site examination? | |
| Not 100% reliable | ||||
| Intersegmental LN clearance (with IOE) | Extensive intersegmental dissection | Allows immediate extension if invasion | Time consuming | Sentinel LN? |
| Precise determination of intersegmental plane | Intrabronchial dye marking | Easy | Inaccurate demarcation line | |
| Cheap | ||||
| Systemic ICG with NIRI | Very accurate | 10% failure rate | ||
| Dedicated system | ||||
| Toxicity if hepatic insufficiency | ||||
| Precise division of intersegmental plane | Stapling | Safe | Difficult handling | Sealing device? |
| Bulky | ||||
| May injure IS vein | ||||
| Cost |
3D, 3-dimensional; IOE, intraoperative examination; LN, lymph nodes; ICG, indocyanine green; NIRI, near-infrared imaging; IS, intersegmental.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor Marcelo F. Jimenez for the series “Precision Surgery for Lung Cancer” published in Precision Cancer Medicine. The article has undergone external peer review.
Conflicts of Interest: The series “Precision Surgery for Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. DG is consultant for an instrument manufacturer (Delacroix Chevalier). DG and ASG are lecturer for Medtronic company. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Howington J, Blum M, Chang A, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:278S-313S.
- Tsutani Y, Tusbokama N, Ito M, et al. Postoperative complications and prognosis after lobar resection versus sublobar resection in elderly patients with clinical stage I non-small lung cancer. Eur J Cardiothorac Surg 2017; [Crossref] [PubMed]
- Rosen J, Hancock J, Kim A, et al. Predictors of mortality after surgical management of lung cancer in the National Cancer Database Ann Thorac Surg 2014;98:1953-60.
- Ginsberg L, Rubinstein L. Randomized trial of lobectomy versus limited resection for T1N0 non-small cell lung cancer. Lung cancer study group. Ann Thorac Surg 1995;60:615-22. [Crossref] [PubMed]
- Whitson BA, Groth SS, Andrade RS, et al. Survival after lobectomy versus segmentectomy for stage I non-small cell lung cancer: a population-based analysis. Ann Thorac Surg 2011;92:1943-50. [Crossref] [PubMed]
- Cao C, Tian DH, Wang D, et al. Sublobar resections—current evidence and future challenges. J Thorac Dis 2017;9:4853-5. [Crossref] [PubMed]
- Aokage K, Saji H, Suzuki K, et al. A non-randomized confirmatory trial of segmentectomy for clinical T1N0 lung cancer with dominant ground glass opacity based on thin-section computed tomography (JCOG1211). Gen Thorac Cardiovasc Surg 2017;65:267-72. [Crossref] [PubMed]
- Nishio W, Yoshimura M, Maniwa Y, et al. Re-assessment of intentional extended segmentectomy for clinical T1aN0 non-small cell lung cancer. Ann Thorac Surg 2016;102:1702-10. [Crossref] [PubMed]
- Koike T, Kitahara A, Sato S. Lobectomy versus segmentectomy in pure solid small-sized non-small cell lung cancer. Ann Thorac Surg 2016;101:1354-60. [Crossref] [PubMed]
- Kodama K, Higashiyama M, Okami J, et al. Oncologic Outcomes of Segmentectomy Versus Lobectomy for Clinical T1a N0 M0 Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;101:504-11. [Crossref] [PubMed]
- Lutz J, Seguin-Givelet A, Grigoroiu M, et al. Oncological results of full thoracoscopic major pulmonary resections for clinical Stage I non-small-cell lung cancer. Eur J Cardiothorac Surg 2019;55:263-70. [Crossref] [PubMed]
- Traibi A, Grigoroiu M, Boulitrop C, et al. Predictive factors for complications of anatomical pulmonary segmentectomies. Interact Cardiovasc Thorac Surg 2013;17:838-44. [Crossref] [PubMed]
- Cao C, Gupta S, Chandrakumar D, et al. Meta-analysis of intentional sublobar resections versus lobectomy for early stage non-small cell lung cancer. Ann Cardiothorac Surg 2014;3:134-41. [PubMed]
- Gossot D, Seguin-Givelet A. Anatomical variations and pitfalls to know during thoracoscopic segmentectomies. J Thorac Dis 2018;10:S1134-44. [Crossref] [PubMed]
- Gossot D. Atlas of endoscopic major pulmonary resections, 2nd edition. Springer-Verlag, 2017.
- Nomori H, Okada M. Illustrated anatomical Segmentectomy for Lung Cancer. Tokyo: Springer-Verlag, 2012.
- Oizumi H, Kanauchi N, Kato H, et al. Anatomic thoracoscopic pulmonary segmentectomy under 3-dimensional multidetector computed tomography simulation: a report of 52 consecutive cases. J Thorac Cardiovasc Surg 2011;141:678-82. [Crossref] [PubMed]
- Eguchi T, Takasuna K, Kitazawa A, et al. Three-dimensional imaging navigation during a lung segmentectomy using an iPad Eur J Cardiothorac Surg 2012;41:893-7. [Crossref] [PubMed]
- Abdelsattar Z, Blackmon S. Using novel technology to augment complex video-assisted thoracoscopic single basilar segmentectomy. J Thorac Dis 2018;10:S1168-78. [Crossref] [PubMed]
- Decaluwe H, Sokolow Y, Deryck F, et al. Thoracoscopic tunnel technique for anatomical lung resections: a 'fissure first, hilum last' approach with staplers in the fissureless patient. Interact Cardiovasc Thorac Surg 2015;21:2-7. [Crossref] [PubMed]
- Gossot D, Grigoroiu M, Brian E, et al. Technical means to improve image quality during thoracoscopic procedures. J Vis Surg 2017;3:53-60. [Crossref] [PubMed]
- Schuchert M, Pettiford B, Keeley S, et al. Anatomic Segmentectomy in the Treatment of Stage I Non-Small Cell Lung Cancer. Ann Thorac Surg 2007;84:926. [Crossref] [PubMed]
- Iwano S, Yokoi K, Taniguchi T, et al. Planning of segmentectomy using three-dimensional computed tomography angiography with a virtual safety margin: technique and initial experience. Lung Cancer 2013;81:410-5. [Crossref] [PubMed]
- Gossot D, Lutz JA, Grigoroiu M, et al. Unplanned Procedures During Thoracoscopic Segmentectomies. Ann Thorac Surg 2017;104:1710-7. [Crossref] [PubMed]
- Wolf AS, Richards WG, Jaklitsch MT, et al. Lobectomy Versus Sublobar Resection for Small (2 cm or Less) Non–Small Cell Lung Cancers. Ann Thorac Surg 2011;92:1819. [Crossref] [PubMed]
- Cox ML, Yang CJ, Speicher PJ, et al. The Role of Extent of Surgical Resection and Lymph Node Assessment for Clinical Stage I Pulmonary Lepidic Adenocarcinoma: An Analysis of 1991 Patients. J Thorac Oncol 2017;12:689-96. [Crossref] [PubMed]
- Tarumi S, Misaki N, Kasai Y, et al. Clinical trial of video-assisted thoracoscopic segmentectomy using infrared thoracoscopy with indocyanine green. Eur J Cardiothorac Surg 2014;46:112-5. [Crossref] [PubMed]
- Guigard S, Triponez F, Bédat B, et al. Usefulness of near-infrared angiography for identifying the intersegmental plane and vascular supply during video-assisted thoracoscopic segmentectomy. Interact Cardiovasc Thorac Surg 2017;25:703-9. [Crossref] [PubMed]
- Pischik VG, Kovalenko A. The role of indocyanine green fluorescence for intersegmental plane identification during video-assisted thoracoscopic surgery segmentectomies. J Thorac Dis 2018;10:S3704-11. [Crossref] [PubMed]
- Seguin-Givelet A, Grigoroiu M, Brian E, et al. Planning and marking for thoracoscopic anatomical segmentectomies. J Thorac Dis 2018;10:S1187-94. [Crossref] [PubMed]
- Sekine Y, Itoh T, Toyoda T, et al. Precise Anatomical Sublobar Resection Using a 3D Medical Image Analyzer and Fluorescence-Guided Surgery With Transbronchial Instillation of Indocyanine Green. Semin Thorac Cardiovasc Surg 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Zhang Z, Liao Y, Ai B, et al. Methylene blue staining: a new technique for identifying intersegmental planes in anatomic segmentectomy. Ann Thorac Surg 2015;99:238-42. [Crossref] [PubMed]
- Sato M, Murayama T, Nakajima J. Techniques of stapler-based navigational thoracoscopic segmentectomy using virtual assisted lung mapping (VAL-MAP). J Thorac Dis 2016;8:S716-30. [Crossref] [PubMed]
- Asakura K, Izumi Y, Kohno M, et al. Effect of cutting technique at the intersegmental plane during segmentectomy on expansion of the preserved segment: comparison between staplers and scissors in ex vivo pig lung. Eur J Cardiothorac Surg 2011;40:e34-8. [Crossref] [PubMed]
- Tao H, Tanaka T, Hayashi T, et al. Influence of stapling the intersegmental planes on lung volume and function after segmentectomy. Interact CardioVasc Thorac Surg 2016;23:548-52. [Crossref] [PubMed]
- Nakano T, Endo S, Mitsuda S, et al. Kyobu Geka 2011;64:792-5. [Stump consolidation after video-assisted thoracoscopic segmentectomy]. [PubMed]
- Miyasaka Y, Oh S, Takahashi N, et al. Postoperative complications and respiratory function following segmentectomy of the lung - comparison of the methods of making an inter-segmental plane. Interact Cardiovasc Thorac Surg 2011;12:426-9. [Crossref] [PubMed]
- Matsumura Y, Okada Y, Shimada K. New surgical technique of pulmonary segmentectomy by ultrasonic scalpel and absorbable sealing materials. Kyobu Geka 2004;57:31-7. [PubMed]
- Ojanguren A, Gossot D, Seguin-Givelet A. Division of the intersegmental plane during thoracoscopic segmentectomy: is stapling an issue? J Thorac Dis 2016;8:2158-64. [Crossref] [PubMed]
- Matsumoto M, Shirahashi K, Yamamoto H. Division of the intersegmental plane using electrocautery for segmentectomy in clinical stage I non-small cell lung cancer. J Thorac Dis 2018;10:S1215-21. [Crossref] [PubMed]
- Okada M, Mimura T, Ikegaki J, et al. A novel video-assisted anatomic segmentectomy technique: selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg 2007;133:753-8. [Crossref] [PubMed]
- Ohtsuka T, Goto T, Anraku M, et al. Dissection of lung parenchyma using electrocautery is a safe and acceptable method for anatomical sublobar resection. J Cardiothorac Surg 2012;7:42-6. [Crossref] [PubMed]
- Gossot D, Merlusca G, Tudor A, et al. Pitfalls related to the use of endostaplers during video-assisted thoracic surgery. Surg Endosc 2009;23:189-92. [Crossref] [PubMed]
Cite this article as: Gossot D, Potenza R, Ojanguren A, Seguin-Givelet A. How to improve the precision of closed chest sublobar resections. Precis Cancer Med 2019;2:14.