
Hanging in the balance: mitochondrial uncoupling protein-2 and the tumor microenvironment
Tumor microenvironment is an ecosystem in which cancer initiation, progression and dissemination take place through complex cell-cell interactions, more or less successfully countered by innate and adaptive immunity of the host (1,2). Outcomes of anti-cancer immune responses greatly depend on recognition of tumor differentiation antigens and destruction of cancer cells by cytotoxic CD8+ T cells recruited into the tumor tissue and activated by dendritic cells. To prevent indiscriminate activation of immunosurveillance, these mechanisms are further regulated by checkpoints involving a variety of molecules such as the programmed cell death protein 1 (PD-1) receptor and its ligand PD-L1 (3). Cancer cells often co-opt immune checkpoints to evade host surveillance, which explains why inhibition of this regulatory system is a promising chapter of cancer research (4). Lack of intratumoral infiltration by CD8+ T cells is a predictor of primary resistance to immune checkpoint blockade therapy in difficult-to-treat malignancies such as melanoma (5). However, the molecular mechanisms by which anti-cancer immune responses can be enhanced are incompletely understood.
In a recent work, Cheng et al. identified the mitochondrial uncoupling protein UCP2 as one of the molecular regulators of anti-cancer immune response (6). Uncoupling proteins belong to the SLC25 group of solute carrier family of transporters (7). UCP1, the prototype uncoupling protein, is restricted to brown adipose tissue where it is abundant and regulates non-shivering thermogenesis by increasing the permeability of mitochondrial inner membrane for protons and dissipating heat from the metabolic energy of electron transport (8). By contrast, UCP2 is a scarce but ubiquitous protein with prominent presence in the immune system exerting biological functions that remain debated (9). There is evidence that UCP2 mediates proton leak when activated by increased levels of intracellular reactive oxygen species (ROS), acting therefore as a sensor and regulator of intracellular oxidative stress in a variety of cell types (10). UCP2 may also interfere with the efficiency of oxidative phosphorylation and modulate the rate of mitochondrial ATP synthesis (11). Moreover, UCP2 has been implicated in mitochondrial utilization of fatty acids and pyruvate (12) and in mitochondrial calcium uptake (13), transport activities that do not necessarily involve corresponding proton leak.
By analyzing tumor-associated gene expression scores in a Swiss cohort of patients who underwent resection of primary cutaneous melanoma, Cheng et al. found that tumor tissue UCP2 expression positively correlated with intratumoral inflammation, CD8+ T cell infiltration and responsiveness to anti-cancer immunotherapy, translating into prolonged survival rates (6). Tumor tissue UCP2 expression was not related to the number of mutations, which led the authors to conclude that augmented T cell responses in these patients were unlikely to result from increased neo-antigen burden (6). Since earlier studies of single-cell mRNA sequencing in melanoma indicated that tumor tissue UCP2 is most abundant in lymphocytes (14), UCP2 may plausibly serve as a marker of intratumoral T cell infiltration. Importantly, Cheng et al. also found that UCP2 abundance in melanoma correlated with the activation of genes controlling IFN-γ signaling, migration of dendritic cells and T cell recruitment as well as with higher PD-L1 expression, all consistent with increased efficiency of immune checkpoint blockade therapy (6).
Based on these correlative observations, Cheng et al. set out to study the impact of UCP2 on tumor progression in allografts generated by using a doxycycline-inducible expression system in B16-OVA and YUMM1.7 melanoma cell lines (6). They found that enforced UCP2 expression thwarted in vivo tumor growth, promoted intratumoral infiltration with CD8+ T cells and NK cells, and resulted in increased pro-inflammatory cytokine production and normalization of tumor microvasculature (6). Absent effect of UCP2 overexpression on tumor growth seen in Rag−/− and Batf3−/− mice respectively confirmed that UCP2-mediated benefits require the presence of dendritic cells and lymphocytes (6). Induction of UCP2 in mice by the commercially available antidiabetic drug PPAR-γ agonist rosiglitazone also sensitized tumor allografts to anti-PD-1 therapy, indicating that pharmacologic stimulation of UCP2 was able to recapitulate the effects of genetically induced UCP2 overexpression (6). Importantly, UCP2 overexpressing allografts showed no change in their intracellular and mitochondrial ROS content or hypoxia-inducible factor 1α (HIF-1α) expression (6), while these molecular events were previously associated with UCP2 action (15,16). Moreover, Cheng et al. found that anti-tumor effects of UCP2 did not involve the β-catenin pathway or PGE2 production, mechanisms known to promote immune evasion of tumor cells (6).
The work of Cheng et al. adds an exciting and potentially game-changing facet to the biology of UCP2 (6). However, the findings seem to generate more questions than provide answers. Effects of UCP2 overexpression in the experimental models utilized were independent of several common oncogenic pathways and apparently did not involve previously established biological functions of mitochondrial uncoupling proteins. Thus, the molecular mechanisms by which UCP2 may enhance anti-tumor immune responses remain unclear. To be fair, controversies about the role of UCP2 in health and disease abound since its identification over 20 years ago. The primary substrate of UCP2-mediated transport (protons, fatty acids, pyruvate, calcium or their combination) remains unknown (9,17-19). Moreover, outcomes of UCP2 actions are sometimes difficult to categorize since it is not always clear how UCP2 would contribute to pathology (Figure 1).
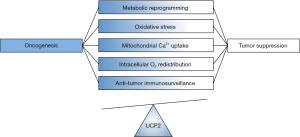
The relationship between UCP2 and mitochondrial ROS is a case in point. ROS are known to have Janus-type biological effects, which range from promoting aberrant cell growth to promoting cell destruction (20,21). Accordingly, cellular responses to ROS levels altered by too much or too little UCP2 may widely differ depending on the clinical or experimental setting. A similar argument can be made for UCP2-induced changes in mitochondrial membrane potential and rates of oxidative phosphorylation (22,23). It is also noteworthy that some of the earliest observations about the biology of UCP2 were made on immune cells which, once made UCP2-deficient, developed enhanced anti-infection and immune properties via increased intracellular ROS levels (24,25). How changes in the ROS content of tumor-infiltrating CD8+ T cells may affect their activity in relation to their UCP2 expression is not clear.
The findings of Cheng et al. are consistent with reports on cell lines derived from highly aggressive cancers such as melanoma, pancreas adenocarcinoma and glioblastoma whereby UCP2 overexpression resulted in repressed malignant phenotypes, reversed metabolic reprogramming and reduced HIF stabilization with no impact on ROS levels (16). However, current findings are more difficult to reconcile with some other studies on the role of UCP2 in cancer. Clinical and experimental studies described higher UCP2 expression in several types of cancer cells (26,27). Also, increased UCP2 mRNA levels have been associated with lower mitochondrial membrane potential, lower intracellular ROS levels, and chemoresistance in melanoma and leukemia cells (28). Moreover, UCP2 overexpression promoted chemoresistance in leukemia and colon cancer cells (29,30). By contrast, inhibition of UCP2 by genetic or pharmacologic approaches made many types of cancer cells (albeit not melanoma) less resistant to different anti-cancer drugs (31). Potential molecular mechanisms implicated in UCP2-mediated protection of cancer cells include reduced intracellular ROS production and reduced apoptosis rates, altered post-translational modification of p53 resulting in weaker tumor surveillance, augmented intracellular oxygen gradients between cytosol and mitochondria promoting HIF stabilization, and induction of the Warburg effect and metabolic reprogramming (29,30,32).
Differences in the biology of cancer cells according to stages of neoplastic development add another layer of complexity when we consider the balance between pro- and anti-tumorigenic effects of UCP2. Low UCP2 expression is associated with increased invasiveness in lung cancer cell lines in vitro and predicts poor response to chemotherapy in lung cancer patients (33). Interestingly, low UCP2-expressing lung cancer cells respond with increased ROS generation in response to the anti-cancer drug paclitaxel (33). Increased intracellular ROS levels in cancer cells stimulate oncogenic pathways, increase the rate of p53 mutations, and promote autophagy (33,34). Thus, chronic oxidative stress due to relative lack of UCP2 action, rather than protection by UCP2 abundance, may represent a selection pressure to activate novel oncogenic mechanisms and account for chemoresistance.
Finally, it is important to note that studies using biological engineering to overexpress a membrane protein such as the SLC25 transporter UCP2 have inherent challenges as proper targeting is essential to draw proper conclusions on protein functions (35). Thus, making sure that overexpressed UCP2 is inserted into the inner mitochondrial membrane may be necessary before analyzing its biological activities, although mitochondrial fractionation studies to validate correct localization are rarely performed (36). For this reason, the fact that rosiglitazone-induced UCP2 was able to sensitize melanoma cells to anti-PD-1 therapy has particular importance in validating the findings based on enforced UCP2 expression in the study of Cheng et al. (6).
In summary, we have now a novel line of evidence for the biological activity of UCP2 as a regulator of anti-tumor immune response and an enabler of immune checkpoint inhibition therapy. What is the molecular mechanism by which UCP2 exerts these beneficial actions? Can we validate these findings by using genetic and pharmacological inhibitors (i.e., knockout models, genipin, or chromanes)? Are there distinct roles of UCP2 expressed in various types of cells within the tumor microenvironment (i.e., tumor cells vs. immune cells)? Can we achieve similar impact on immune checkpoint blockade in other types of difficult-to-treat cancer? What specific UCP2 activators (beyond rosiglitazone) can we consider to support the anti-tumor immune cycle? Future research will hopefully provide answers to these practical and theoretical questions before stimulation of UCP2 could find its way into clinical applications.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Dr. Lin Tian (Post-doctoral Research Fellow, Cancer Biology and Genetics Program, Memorial Sloan Kettering Cancer Center, New York, NY, USA).
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pcm.2019.04.01). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Noonan DM, De Lerma Barbaro A, Vannini N, et al. Inflammation, inflammatory cells and angiogenesis: decisions and indecisions. Cancer Metastasis Rev 2008;27:31-40. [Crossref] [PubMed]
- Quante M, Varga J, Wang TC, et al. The gastrointestinal tumor microenvironment. Gastroenterology 2013;145:63-78. [Crossref] [PubMed]
- Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol 2012;12:269-81. [Crossref] [PubMed]
- Pardoll D. Cancer and the Immune System: Basic Concepts and Targets for Intervention. Semin Oncol 2015;42:523-38. [Crossref] [PubMed]
- Reticker-Flynn NE, Engleman EG. A gut punch fights cancer and infection. Nature 2019;565:573-4. [Crossref] [PubMed]
- Cheng WC, Tsui YC, Ragusa S, et al. Uncoupling protein 2 reprograms the tumor microenvironment to support the anti-tumor immune cycle. Nat Immunol 2019;20:206-17. [Crossref] [PubMed]
- Palmieri F. The mitochondrial transporter family SLC25: identification, properties and physiopathology. Mol Aspects Med 2013;34:465-84. [Crossref] [PubMed]
- Klingenberg M. Uncoupling protein--a useful energy dissipator. J Bioenerg Biomembr 1999;31:419-30. [Crossref] [PubMed]
- Nedergaard J, Cannon B. The 'novel' uncoupling proteins UCP2 and UCP3: What do they really do? Pros and cons for suggested functions. Exp Physiol 2003;88:65-84. [Crossref] [PubMed]
- Echtay KS, Roussel D, St-Pierre J, et al. Superoxide activates mitochondrial uncoupling proteins. Nature 2002;415:96-9. [Crossref] [PubMed]
- Zhang CY, Baffy G, Perret P, et al. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell 2001;105:745-55. [Crossref] [PubMed]
- Pecqueur C, Bui T, Gelly C, et al. Uncoupling protein-2 controls proliferation by promoting fatty acid oxidation and limiting glycolysis-derived pyruvate utilization. FASEB J 2008;22:9-18. [Crossref] [PubMed]
- Trenker M, Malli R, Fertschai I, et al. Uncoupling proteins 2 and 3 are fundamental for mitochondrial Ca2+ uniport. Nat Cell Biol 2007;9:445-52. [Crossref] [PubMed]
- Tirosh I, Izar B, Prakadan SM, et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 2016;352:189-96. [Crossref] [PubMed]
- Esteves TC, Brand MD. The reactions catalysed by the mitochondrial uncoupling proteins UCP2 and UCP3. Biochim Biophys Acta 2005;1709:35-44. [Crossref] [PubMed]
- Esteves P, Pecqueur C, Ransy C, et al. Mitochondrial retrograde signaling mediated by UCP2 inhibits cancer cell proliferation and tumorigenesis. Cancer Res 2014;74:3971-82. [Crossref] [PubMed]
- Klingenberg M. Uncoupling proteins--how do they work and how are they regulated. IUBMB Life 2001;52:175-9. [Crossref] [PubMed]
- Graier WF, Trenker M, Malli R. Mitochondrial Ca2+, the secret behind the function of uncoupling proteins 2 and 3? Cell Calcium 2008;44:36-50. [Crossref] [PubMed]
- Bouillaud F. UCP2, not a physiologically relevant uncoupler but a glucose sparing switch impacting ROS production and glucose sensing. Biochimica et Biophysica Acta 2009;1787:377-83. [Crossref] [PubMed]
- Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol 2002;192:1-15. [Crossref] [PubMed]
- Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem J 2007;401:1-11. [Crossref] [PubMed]
- Heerdt BG, Houston MA, Augenlicht LH. Potentiation by specific short-chain fatty acids of differentiation and apoptosis in human colonic carcinoma cell lines. Cancer Res 1994;54:3288-93. [PubMed]
- Miwa S, Brand MD. Mitochondrial matrix reactive oxygen species production is very sensitive to mild uncoupling. Biochem Soc Trans 2003;31:1300-1. [Crossref] [PubMed]
- Negre-Salvayre A, Hirtz C, Carrera G, et al. A role for uncoupling protein-2 as a regulator of mitochondrial hydrogen peroxide generation. FASEB J 1999;11:809-15. [Crossref] [PubMed]
- Arsenijevic D, Onuma H, Pecqueur C, et al. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet 2000;26:435-9. [Crossref] [PubMed]
- Horimoto M, Resnick MB, Konkin TA, et al. Expression of uncoupling protein-2 in human colon cancer. Clin Cancer Res 2004;10:6203-7. [Crossref] [PubMed]
- Li W, Nichols K, Nathan CA, et al. Mitochondrial uncoupling protein 2 is up-regulated in human head and neck, skin, pancreatic, and prostate tumors. Cancer Biomark 2013;13:377-83. [Crossref] [PubMed]
- Harper ME, Antoniou A, Villalobos-Menuey E, et al. Characterization of a novel metabolic strategy used by drug-resistant tumor cells. Faseb J 2002;16:1550-7. [Crossref] [PubMed]
- Derdak Z, Mark NM, Beldi G, et al. The mitochondrial uncoupling protein-2 promotes chemoresistance in cancer cells. Cancer Res 2008;68:2813-9. [Crossref] [PubMed]
- Samudio I, Fiegl M, McQueen T, et al. The warburg effect in leukemia-stroma cocultures is mediated by mitochondrial uncoupling associated with uncoupling protein 2 activation. Cancer Res 2008;68:5198-205. [Crossref] [PubMed]
- Baffy G. Mitochondrial uncoupling in cancer cells: Liabilities and opportunities. Biochim Biophys Acta Bioenerg 2017;1858:655-64. [Crossref] [PubMed]
- Hagen T, Taylor CT, Lam F, et al. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1alpha. Science 2003;302:1975-8. [Crossref] [PubMed]
- Su WP, Lo YC, Yan JJ, et al. Mitochondrial uncoupling protein 2 regulates the effects of paclitaxel on Stat3 activation and cellular survival in lung cancer cells. Carcinogenesis 2012;33:2065-75. [Crossref] [PubMed]
- Li ZY, Yang Y, Ming M, et al. Mitochondrial ROS generation for regulation of autophagic pathways in cancer. Biochem Biophys Res Commun 2011;414:5-8. [Crossref] [PubMed]
- Jensen HM, Eng T, Chubukov V, et al. Improving membrane protein expression and function using genomic edits. Sci Rep 2017;7:13030. [Crossref] [PubMed]
- Derdak Z, Garcia TA, Baffy G. Detection of uncoupling protein-2 as a mitochondrial modulator of apoptosis. Methods Mol Biol 2009;559:205-17. [Crossref] [PubMed]
Cite this article as: Baffy G. Hanging in the balance: mitochondrial uncoupling protein-2 and the tumor microenvironment. Precis Cancer Med 2019;2:12.

