
Guanine residues of precursor microRNA’s terminal loop as a potential target for cancer therapy and prevention
Introduction
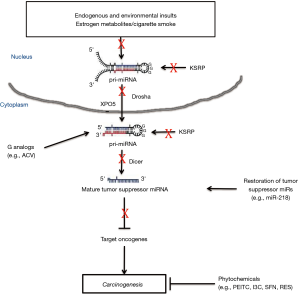
MicroRNAs (miRNAs, miRs) are small endogenous noncoding RNA molecules that negatively regulate gene expression affecting many biological processes and diseases, including cancer (1,2). The active and mature miRNA form is ~22 nucleotides (nt) in length, and is the result of a multiple-step process. This begins with the primary long transcript (pri-miRNA) being processed by an RNase, termed Drosha, and its partner protein DiGeorge syndrome chromosomal region 8 (DGCR8), that cut it into a ~70-nt stem loop (SL) precursor (pre-miRNA), which contains the mature miRNA sequence in one of its arms and the less abundant, partially complementary miRNA mature form, in the other arm (3,4). The pre-miRNA is then actively transported by exportin-5 (XPO5) from the nucleus to the cytoplasm, where it is processed by another RNase, termed Dicer (5,6). The result of this second processing event is a double stranded RNA, where one of its strands is incorporated into the Argonaute (Ago) protein of the RNA-induced silencing complex (RISC) that commonly targets it to a 3' untranslated region (3'UTR) of a specific mRNA and leads to its degradation (1).
The precursor miRNA terminal loop (TL) is an important platform for different RNA-binding proteins (RBPs) which operate as activators or repressors of Drosha and Dicer (7). Two of these RBPs are known to selectivity regulate miRNA processing by binding to guanine (G) residues-enriched motifs in the TL: miRNAs with the sequence motif GGAG in their TL are regulated through the binding of the RBP Lin28, which interferes with Dicer processing (8), while the sequence AGGGU in the TL promotes miRNA maturation by the K-homology splicing regulatory protein (KSRP) RBP (9). It has recently been shown that modification of KSRP results in the downregulation of a subset of TL G-rich miRNAs, subsequently promoting tumorigenesis (10).
The widespread downregulation of miRNA expression is a phenomenon observed in many types of human cancer (11-14). A comprehensive repression of miRNA expression has also been reported after exposure to cigarette-smoke (CS) (15-17), and treatment with the hormone estrogen (17β-estradiol; E2) (18-20). These aforementioned alterations in miRNA expression can occur as a result of changes in the transcription of miRNA genes, as was shown after c-Myc activation (21), miRNA export from the nucleus (22), or at any stage of the miRNA maturation process by modulation of key regulators or components of the miRNA biogenesis pathway, including the microprocessor complex Drosha-DGCR8, and Dicer (23).
Recently, we have found an association between the comprehensive miRNA reduction observed in human cancers and a high TL-G content in their precursors (24,25), as well as a similar G enrichment existing in TLs of downregulated miRNAs after E2 exposure (26). The potential carcinogenic activity of estrogens involves their oxidative metabolism to catechol estrogens and the reactive quinone metabolites forming specific DNA adducts at the N-7 G (27,28). These adducts generate apurinic sites that can be converted into mutations by error-prone repair, which in turn may initiate tumorigenesis (29). Furthermore, oxidative metabolites of estrogens form 8-oxoguanine (8-oxoG); a major product of oxidation damage, which serves as a biomarker of oxidative stress and eventually leads to carcinogenesis (30).
Because G has a lower oxidation potential it is most easily oxidized among the four nucleobases (31). Also, sequences with repeated G bases (GG or GGG) show higher reactivity toward oxidation than isolated G bases (32). Most interestingly, of the different G combinations in TL sequences of both cancer and E2-repressed miRNAs, the relative enrichment of double G (GG) and triple G (GGG) was especially dominant (24-26). Therefore, oxidation and/or adduct formation by carcinogens, such as CS and estrogen metabolites, that react with G/GG/GGG in precursor of tumor suppressor (TS) miRNA TL may contribute to the development of cancer. Herein we suggest several hypotheses and potential ways for the prevention of cancer that may be initiated by interaction of carcinogens with the G content of TS precursor miRNA’s TL.
Cellular pathways used to repair G damage
There are various types of DNA repair mechanisms that specialize in removing different kinds of DNA lesions caused by endogenous and environmental insults, thereby helping to prevent cancer. One example is the base excision repair (BER) system used to repair oxidative lesions caused by reactive oxygen species (ROS) such as 8-oxoG (33,34). Another example is O6-methylguanine DNA methyltransferase (O6MT) that removes O6-alkylguanine adducts and effectively restores the G base in DNA (27).
A number of lines of evidence indicate that there are several possible cellular repair mechanisms to cope with RNA damage (35). For example, it has been previously shown that the repair of damaged bases in RNA can be executed by several members of the AlkB family of enzymes, by a unique oxidative demethylation repair mechanism that removes methyl adducts (36). Thus, activation of such cellular defense mechanisms may represent a possible therapeutic direction for repairing various adducts (37), including potential G adducts in miRNA TLs.
The potential use of G analogs for cancer prevention
Several guanosine analogs, such as acyclovir (ACV), are widely used for the treatment of herpesvirus infections and also as antitumor agents for the combined chemotherapy of cancer (38). These drugs compete with deoxyguanosine triphosphate (dGTP) as a substrate for viral DNA polymerase in herpesvirus-infected human cells, resulting in early chain termination and inhibit virus DNA synthesis and replication (39). ACV is a nucleic acid analog made from guanosine (also known as acycloguanosine), which has incomplete cyclic sugar ribose where the carbons at the 3' and 4' positions are missing, while its G moiety is left intact (40). Therefore, introduction of ACV into cells may cause competition for carcinogens binding with the natural G nucleotides, and may reduce oxidatively-generated damage to cellular RNA and nuclear DNA. Notably, a recent study has shown a potent anti-cancer effect by ACV (41). ACV treatment of MCF7 breast cancer cells decreased their growth and proliferation rate, inhibiting both colony formation ability and cell invasion capacity (41). The exact role of ACV in these anti-cancer effects is currently unknown (41), however, it may potentially involve protection of G-rich TS miRNAs.
Restoration of TL G-enriched TS miRNA expression
As mentioned above, the global miRNA repression observed in cancer has been found to be associated with G enrichment in the TLs of their precursors. Whether this repression is caused by the carcinogen’s effects on the miRNA processing machinery (42), or through changes in the expression or deletion of miRNA encoding genes (21,43), the result is the downregulation of TS miRs (e.g., let-7, miR-34) which probably contributes to neoplastic transformation by allowing an increased expression of their target oncogenes [e.g., Kirsten rat sarcoma viral oncogene homolog (KRAS), epidermal growth factor receptor (EGFR)]. Intriguingly, we have recently revealed that the pre-miRNAs TLs of TS miRs are predominantly G enriched (44).
A therapeutic approach for systemically delivering synthetic TS miR mimics have been demonstrated, including several studies showing that restoring the oncosuppressor activity of miR-34a can successfully inhibit tumor growth (45,46). Interestingly, miR-34a precursor TL has a relatively high G content (35% G enrichment). Another potential candidate for such an intervention is miR-218, which is downregulated and acts as a TS miR in various types of human cancers, including lung cancer (47), where it is shown to inhibit cell proliferation and migration by targeting the EGFR oncogene (48). MiR-218 is the most differentially expressed miRNA in the airway epithelium of smokers, with its expression being 4-fold down-regulated (16). Its predicted targets, such as V-Maf Avian Musculoaponeurotic Fibrosarcoma Oncogene Homolog G (MAFG), EGFR-coamplified and overexpressed protein (ECOP), and LIM and SH3 protein 1 (LASP-1), are significantly overrepresented among the genes upregulated in the bronchial epithelium of smokers (49). Moreover, the pre-miRNA’s TL of miR-218 is remarkably G-enriched (43% G enrichment) (44).
Fruits and vegetables and their phytochemicals used for cancer chemoprevention
The preventative and therapeutic effects of using fruit and vegetables and their dietary phytochemicals against various types of cancer are well documented (50,51). Amongst them, cruciferous vegetables have been extensively studied and are especially known for their cancer chemopreventive compounds; phenethyl isothiocyanate (PEITC), sulforaphane (SFN), and indole-3-carbinol (I3C) (52,53). Administration of PEITC and I3C attenuated the CS-induced downregulation of miRNAs (54), which were shown to have a high G content in their TLs (42). Furthermore, PEITC was shown to significantly inhibit the formation of the xenoestrogen bisphenol A (BPA)-induced DNA adducts in mice (55).
Another compound with known antioxidant and chemopreventive activities is the dietary polyphenol derived from grapes, Resveratrol (RES) (56). Multiple studies have shown that RES prevents cancer initiation by blocking oxidation of catechol estrogens to their quinones and estrogen-DNA adducts formation (57-60). Further, both RES and SFN were shown to induce protective phase II enzymes activity, resulting in reduction of estrogen-induced DNA damage (61). Thus, increasing fruits and vegetables (including cruciferous) intake in the diet seems to be a simple and effective way for cancer prevention (62).
Conclusions
Endogenous and exogenous carcinogens may oxidize and form adducts at the G (especially GG and GGG) content of TS miRNAs TLs. The resulted G lesions may cause extensive repression of TS miRNAs, leading to the induction of their target oncogenes and carcinogenesis, while several potential methods may be used for its prevention (Figure 1). Once the molecular mechanisms of global miRNA downregulation during tumorigenesis is fully elucidated, it can lead to the development of novel strategies for combating cancer. Revealing the role of G content of precursor miRNA’s TL in these processes appears to be a promising direction towards this goal.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pcm.2019.01.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009;136:215-233. [Crossref] [PubMed]
- Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol 2014;9:287-314. [Crossref] [PubMed]
- Zeng Y, Yi R, Cullen BR. Recognition and cleavage of primary microRNA precursors by the nuclear processing enzyme Drosha. EMBO J 2005;24:138-48. [Crossref] [PubMed]
- Yang JS, Phillips MD, Betel D, et al. Widespread regulatory activity of vertebrate microRNA* species. RNA 2011;17:312-26. [Crossref] [PubMed]
- Lund E, Güttinger S, Calado A, et al. Nuclear export of microRNA precursors. Science 2004;303:95-8. [Crossref] [PubMed]
- Cullen BR. Transcription and processing of human microRNA precursors. Mol Cell 2004;16:861-5. [Crossref] [PubMed]
- Libri V, Miesen P, van Rij RP, et al. Regulation of microRNA biogenesis and turnover by animals and their viruses. Cell Mol Life Sci 2013;70:3525-44. [Crossref] [PubMed]
- Heo I, Joo C, Kim YK, et al. TUT4 in concert with Lin28 suppresses microRNA biogenesis through pre-microRNA uridylation. Cell 2009;138:696-708. [Crossref] [PubMed]
- Trabucchi M, Briata P, Garcia-Mayoral M, et al. The RNA-binding protein KSRP promotes the biogenesis of a subset of microRNAs. Nature 2009;459:1010-4. [Crossref] [PubMed]
- Yuan H, Deng R, Zhao X, et al. SUMO1 modification of KHSRP regulates tumorigenesis by preventing the TL-G-Rich miRNA biogenesis. Mol Cancer 2017;16:157. [Crossref] [PubMed]
- Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature 2005;435:834-8. [Crossref] [PubMed]
- Ozen M, Creighton CJ, Ozdemir M, et al. Widespread deregulation of microRNA expression in human prostate cancer. Oncogene 2008;27:1788-93. [Crossref] [PubMed]
- Dvinge H, Git A, Gräf S, et al. The shaping and functional consequences of the microRNA landscape in breast cancer. Nature 2013;497:378-82. [Crossref] [PubMed]
- Cohen A, Burgos-Aceves MA, Smith Y. Estrogen repression of microRNA as a potential cause of cancer. Biomed Pharmacother 2016;78:234-8. [Crossref] [PubMed]
- Izzotti A, Calin GA, Arrigo P, et al. Downregulation of microRNA expression in the lungs of rats exposed to cigarette smoke. FASEB J 2009;23:806-12. [Crossref] [PubMed]
- Schembri F, Sridhar S, Perdomo C, et al. MicroRNAs as modulators of smoking-induced gene expression changes in human airway epithelium. Proc Natl Acad Sci U S A 2009;106:2319-24. [Crossref] [PubMed]
- Graff JW, Powers LS, Dickson AM, et al. Cigarette smoking decreases global microRNA expression in human alveolar macrophages. PLoS One 2012;7:e44066. [Crossref] [PubMed]
- Maillot G, Lacroix-Triki M, Pierredon S, et al. Widespread estrogen-dependent repression of micrornas involved in breast tumor cell growth. Cancer Res 2009;69:8332-40. [Crossref] [PubMed]
- Yu X, Zhang X, Dhakal IB, et al. Induction of cell proliferation and survival genes by estradiol-repressed microRNAs in breast cancer cells. BMC Cancer 2012;12:29. [Crossref] [PubMed]
- Cohen A, Smith Y. Estrogen regulation of microRNAs, target genes, and microRNA expression associated with vitellogenesis in the zebrafish. Zebrafish 2014;11:462-78. [Crossref] [PubMed]
- Chang TC, Yu D, Lee YS, et al. Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet 2008;40:43-50. [Crossref] [PubMed]
- Sun HL, Cui R, Zhou J, et al. ERK Activation Globally Downregulates miRNAs through Phosphorylating Exportin-5. Cancer Cell 2016;30:723-36. [Crossref] [PubMed]
- Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer 2015;15:321-33. [Crossref] [PubMed]
- Cohen A, Burgos-Aceves MA, Smith Y. microRNAs downregulation in cancer is associated with guanine enrichment in the terminal loop sequences of their precursors. MicroRNA 2018;7:20-7. [Crossref] [PubMed]
- Cohen A, Burgos-Aceves MA, Smith Y. Global microRNA downregulation: all roads lead to estrogen. J Xiangya Med 2017;2:59. [Crossref]
- Cohen A, Burgos-Aceves MA, Kahan T, et al. Estrogen repression of microRNAs is associated with high guanine content in the terminal loop sequences of their precursors. Biomedicines 2017;5:3. [Crossref] [PubMed]
- Boysen G, Pachkowski BF, Nakamura J, et al. The formation and biological significance of N7-guanine adducts. Mutat Res 2009;678:76-94. [Crossref] [PubMed]
- Cavalieri EL, Rogan EG. Depurinating estrogen-DNA adducts, generators of cancer initiation: their minimization leads to cancer prevention. Clin Transl Med 2016;5:12. [Crossref] [PubMed]
- Cavalieri E, Chakravarti D, Guttenplan J, et al. Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention. Biochim Biophys Acta 2006;1766:63-78. [PubMed]
- Słowikowski BK, Lianeri M, Jagodziński PP. Exploring estrogenic activity in lung cancer. Mol Biol Rep 2017;44:35-50. [Crossref] [PubMed]
- Kawanishi S, Hiraku Y, Oikawa S. Mechanism of guanine-specific DNA damage by oxidative stress and its role in carcinogenesis and aging. Mutat Res 2001;488:65-76. [Crossref] [PubMed]
- Senthilkumar K, Grozema FC, Guerra CF, et al. Mapping the sites for selective oxidation of guanines in DNA. J Am Chem Soc 2003;125:13658-9. [Crossref] [PubMed]
- Torgovnick A, Schumacher B. DNA repair mechanisms in cancer development and therapy. Front Genet 2015;6:157. [Crossref] [PubMed]
- Delaney S, Jarem DA, Volle CB, et al. Chemical and biological consequences of oxidatively damaged guanine in DNA. Free Radic Res 2012;46:420-41. [Crossref] [PubMed]
- Feyzi E, Sundheim O, Westbye MP, et al. RNA base damage and repair. Curr Pharm Biotechnol 2007;8:326-31. [Crossref] [PubMed]
- Yang CG, Yi C, Duguid EM, et al. Crystal structures of DNA/RNA repair enzymes AlkB and ABH2 bound to dsDNA. Nature 2008;452:961-5. [Crossref] [PubMed]
- Chen F, Tang Q, Bian K, et al. Adaptive Response Enzyme AlkB Preferentially Repairs 1-Methylguanine and 3-Methylthymine Adducts in Double-Stranded DNA. Chem Res Toxicol 2016;29:687-93. [Crossref] [PubMed]
- De Clercq E. Guanosine analogues as anti-herpesvirus agents. Nucleosides Nucleotides Nucleic Acids 2000;19:1531-41. [Crossref] [PubMed]
- Klysik K, Pietraszek A, Karewicz A, et al. Acyclovir in the Treatment of Herpes Viruses - a Review. Curr Med Chem 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Sacks SL, Straus SE, Whitley RJ, et al. editors. Clinical management of herpes viruses. Washington, D.C: IOS Press, 1995.
- Shaimerdenova M, Karapina O, Mektepbayeva D, et al. The effects of antiviral treatment on breast cancer cell line. Infect Agent Cancer 2017;12:18. [Crossref] [PubMed]
- Izzotti A, Pulliero A. The effects of environmental chemical carcinogens on the microRNA machinery. Int J Hyg Environ Health 2014;217:601-27. [Crossref] [PubMed]
- Calin GA, Sevignani C, Dumitru CD, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A 2004;101:2999-3004. [Crossref] [PubMed]
- Cohen A, Burgos-Aceves MA, Smith Y. Guanine content of microRNAs is associated with their tumor suppressive and oncogenic roles in lung and breast cancers. 2019. Available online: https://www.biorxiv.org/content/10.1101/518472v1
- Trang P, Wiggins JF, Daige CL, et al. Systemic delivery of tumor suppressor microRNA mimics using a neutral lipid emulsion inhibits lung tumors in mice. Mol Ther 2011;19:1116-22. [Crossref] [PubMed]
- Ofek P, Calderón M, Mehrabadi FS, et al. Restoring the oncosuppressor activity of microRNA-34a in glioblastoma using a polyglycerol-based polyplex. Nanomedicine 2016;12:2201-14. [Crossref] [PubMed]
- Cohen A, Burgos-Aceves MA, Smith Y. A potential role for estrogen in cigarette smoke-induced microRNA alterations and lung cancer. Transl Lung Cancer Res 2016;5:322-30. [Crossref] [PubMed]
- Zhu K, Ding H, Wang W, et al. Tumor-suppressive miR-218-5p inhibits cancer cell proliferation and migration via EGFR in non-small cell lung cancer. Oncotarget 2016;7:28075-85. [PubMed]
- Perdomo C, Spira A, Schembri F. MiRNAs as regulators of the response to inhaled environmental toxins and airway carcinogenesis. Mutat Res 2011;717:32-7. [Crossref] [PubMed]
- Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer 2003;3:768-80. [Crossref] [PubMed]
- Gullett NP, Ruhul Amin AR, Bayraktar S, et al. Cancer prevention with natural compounds. Semin Oncol 2010;37:258-81. [Crossref] [PubMed]
- Murillo G, Mehta RG. Cruciferous vegetables and cancer prevention. Nutr Cancer 2001;41:17-28. [Crossref] [PubMed]
- Herr I, Büchler MW. Dietary constituents of broccoli and other cruciferous vegetables: implications for prevention and therapy of cancer. Cancer Treat Rev 2010;36:377-83. [Crossref] [PubMed]
- Izzotti A, Calin GA, Steele VE, et al. Chemoprevention of cigarette smoke-induced alterations of MicroRNA expression in rat lungs. Cancer Prev Res (Phila) 2010;3:62-72. [Crossref] [PubMed]
- Izzotti A, Kanitz S, D'Agostini F, et al. Formation of adducts by bisphenol A, an endocrine disruptor, in DNA in vitro and in liver and mammary tissue of mice. Mutat Res 2009;679:28-32. [Crossref] [PubMed]
- Savouret JF, Quesne M. Resveratrol and cancer: a review. Biomed Pharmacother 2002;56:84-7. [Crossref] [PubMed]
- Zahid M, Gaikwad NW, Rogan EG, et al. Inhibition of depurating estrogen-DNA adduct formation by natural compounds. Chem Res Toxicol 2007;20:1947-53. [Crossref] [PubMed]
- Lu F, Zahid M, Wang C, et al. Resveratrol prevents estrogen-DNA adduct formation and neoplastic transformation in MCF-10F cells. Cancer Prev Res (Phila) 2008;1:135-45. [Crossref] [PubMed]
- Hinrichs B, Zahid M, Saeed M, et al. Formation of diethylstilbestrol-DNA adducts in human breast epithelial cells and inhibition by resveratrol. J Steroid Biochem Mol Biol 2011;127:276-81. [Crossref] [PubMed]
- Cavalieri EL, Rogan EG. Depurinating estrogen-DNA adducts in the etiology and prevention of breast and other human cancers. Future Oncol 2010;6:75-91. [Crossref] [PubMed]
- Yager JD. Mechanisms of estrogen carcinogenesis: The role of E2/E1-quinone metabolites suggests new approaches to preventive intervention-A review. Steroids 2015;99:56-60. [Crossref] [PubMed]
- Bosetti C, Filomeno M, Riso P, et al. Cruciferous vegetables and cancer risk in a network of case-control studies. Ann Oncol 2012;23:2198-203. [Crossref] [PubMed]
Cite this article as: Cohen A, Burgos-Aceves MA, Smith Y. Guanine residues of precursor microRNA’s terminal loop as a potential target for cancer therapy and prevention. Precis Cancer Med 2019;2:2.

