
What is the best drug as a front-line treatment for EGFR activating mutation?
Introduction
Lung cancer is the leading cause of cancer related mortality (1,2). There are two reasons for this: first, lung cancer cells easily metastasize, so when lung cancer is detected, it is frequently found at an advanced stage. Second, the recurrence rate after compete resection is relatively high (3), even in early-stage cases (4). The essential qualities of a poor prognosis are ascribed to the diversity of gene mutations (5). Among the identified gene alterations, the epidermal growth factor receptor (EGFR) mutation is the best studied, and its inhibitors are currently deemed essential for the treatment of lung cancer.
This review discusses intratumor heterogeneity, compares the effects EGFR-tyrosine kinase inhibitors (TKIs), and evaluates the outcomes of head-to-head trials of EGFR-TKIs, considers the overall survival (OS) and effect of uncommon/compound EGFR mutations, and describes the current perspectives regarding first-line treatment and molecular-targeted drugs for patients with EGFR mutations.
Intratumor heterogeneity
In the initial stage, driver mutations trigger cancer development. Through the process of proliferation, various gene alterations are therefore known to occur (6). In the process, tumor tissue changes into various types of transforming cells. Therefore, simple EGFR mutations, such as Del19or L858R, simultaneously occur along with the various changes in tumor tissue at specific frequencies (7).
Comparisons of EGFR-TKIs
So-called “first-generation” EGFR-TKIs, such as gefitinib/erlotinib, are reversible competitors for adenosine triphosphate (ATP) binding sites with a quinazoline circle (Figure 1). As the targeting molecule is only EGFR (ErbB1) (8), the general term of reversible EGFR-TKIs has been applied (9). In contrast, “second-generation” EGFR-TKIs, such as afatinib, are irreversible competitors for ATP binding sites. These agents are aniline-quinazoline derivatives that covalently bind to specific catalytic sites of different members of the ErbB receptor family, including EGFR (ErbB1) as well as ErbB2 and ErbB4, and block the transphosphorylation of ErbB3 in order to inhibit all ERBB family signaling (10). Thus, agents such as afatinib have been given the generic name of irreversible ErbB family blockers (10).
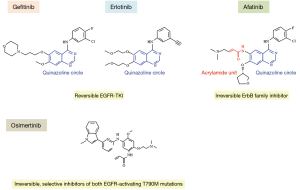
Of note, afatinib reportedly improved the progression-free survival (PFS), OS and disease control rate (DCR) compared with erlotinib (11). EGFR mutations are rare (<5%) in squamous cell carcinoma (SQ) of the lung (12). However, around 5–20% of SQ cases express HER2, with substantial overexpression (13), and roughly 30% of them overexpress HER3 (14). Furthermore, genetic aberrations in HER2 and HER3 in several signaling molecules downstream of the ErbB receptors have been identified in SQ (12). These findings show that afatinib not only targets EGFR but is also an irreversible ErbB family blocker.
“Third-generation” EGFR-TKIs, such as osimertinib, have been developed, showing high potency for T790M mutation-positive tumors (15). Osimertinib exerts irreversible covalent binding to mutant EGFR (16).
Head-to-head trials of EGFR-TKIs
Five pivotal studies have compared the outcomes with several EGFR-TKIs in head-to-head trials for patients with EGFR mutations (Table 1). Direct comparisons of first-generation EGFR-TKIs showed a reasonable long-term survival equivalent to the outcomes with gefitinib/erlotinib. Thus, gefitinib exerts virtually the same clinical effect as erlotinib, with no marked differences noted among first-generation EGFR-TKIs. However, the second-generation EGFR-TKI afatinib might offer improved efficacy compared with gefitinib (17-19), as afatinib has also shown greater anticancer activity than other reversible EGFR-TKIs as mentioned above. This suggests that first- and second-generation EGFR-targeted drugs might not be interchangeable (19,20). Furthermore, the length of the survival for the patients treated with the third-generation agent osimertinib was longer than that for those treated by first-generation EGFR-TKIs (21). The clinical usefulness of third-generation EGFR-TKIs is thus considerably superior to that of first-generation EGFR-TKIs. At present, there are no data available regarding the direct comparison of second- and third-generation EGFR-TKIs.
Table 1
| Generation TKI | Trial | TKI | Line | PFS (months) (HR, P value) | OS (months) (HR, P value) |
|---|---|---|---|---|---|
| 1st vs. 1st | WJOG 5108L | G vs. E | 2nd | 8.3 vs. 10.0; HR: 1.093, P=0.424 | 26.5 vs. 31.4; HR, 1.189, P=0.221 |
| 1st vs. 1st | CTONG 0901 | G vs. E | All | 10.4 vs. 13.0; HR, 0.81, P=0.108 | 20.1 vs. 22.9; HR, 0.84, P=0.250 |
| 2nd vs. 1st | LUX-Lung 7 | A vs. G | 1st | 11.0 vs. 10.9; HR, 0.74; P=0.0178 | 27.9 vs. 24.5; HR, 0.86, P=0.2580 |
| 2nd vs. 1st | ARCHER 1050 | D vs. G | 1st | 14.7 vs. 9.2; HR, 0.59, P<0.0001 | 34.1 vs. 26.8; HR, 0.76, P 0.0438 |
| 3rd vs. 1st | FLAURA | O vs. G/E | 1st | 18.9 vs. 10.2; HR: 0.46, P<0.001 | Not reported |
EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; PFS, progression-free survival; HR, hazard ratio; OS, overall survival; G, gefitinib; E, erlotinib; A, afatinib; D, dacomitinib; O, osimertinib.
Consideration of the OS
Clinical trials have shown that the OS with first-generation EGFR-TKIs was 19.3–36.3 months (22-26). In contrast, the OS in Japanese patients treated with the second-generation afatinib was reportedly 41.7–46.9 months (27), suggesting that the PFS is longer in patients treated with second-generation EGFR-TKIs than in those treated with first-generation EGFR-TKIs.
Effects of uncommon EGFR mutations
EGFR mutations are loosely grouped into common and uncommon (minor) mutations (28). The two most common EGFR mutations, EGFR exon 19 deletion (del19) and the Leu858Arg point mutation in exon 21 (L858R), account for roughly 90% of all mutation-positive, non-small cell lung cancer (NSCLC) tumors and are sensitive to drugs that target EGFR (29). The remaining 10% of EGFR mutations fall into a heterogeneous group of molecular alterations (uncommon mutations) with variable responses to EGFR-targeted drugs (30). An in vitro study showed that uncommon EGFR mutations are generally insensitive to gefitinib and erlotinib but are sensitive to afatinib and osimertinib (31). Indeed, gefitinib and erlotinib are not be not expected to imbue any further survival improvement in patients with uncommon EGFR mutations (32,33). However, afatinib induced clinical shrinkage in patients with uncommon EGFR mutations. Furthermore, the PFS and OS were quite favorable, with a high response rate (RR) (30).
Interestingly, a phase II study conducted in Japan in a population with EGFR-mutation-positive NSCLC showed the modest but noteworthy efficacy of afatinib, with a median PFS of 4.4 months and an RR of 8.2%, in third- and fourth-line patients with NSCLC who had acquired resistance to first-generation EGFR-TKIs (34). These reasons might explain the efficacy of afatinib against uncommon and compound EGFR mutations (described below).
Effects of compound EGFR mutation
Rare EGFR mutations are expected to be more frequently encountered with the advent of more sensitive and precise tumor genotyping systems (35). Compound EGFR mutations, defined as double or multiple mutations in the EGFR-TK domain, are being more frequently detected with advances in sequencing technology, but their clinical significance is unclear (36).
We previously reported the frequency of compound EGFR mutations to be around 20% (28). This frequency is consistent with the findings of previous studies (31,36). In an in vitro study, the sensitivity of first-generation EGFR-TKIs was low for tumor cells harboring compound EGFR mutations. In contrast, that of afatinib was very high (31). A patient harboring complex exon 18 G719X and exon 20 S768I mutations started to receive afatinib and has exhibited a good response without progression for one year (37). Saxon et al. reported that first- and third-generation TKIs exhibited a decreased capacity to prevent EGFR phosphorylation in EGFR L858M/L861Q cells compared with cells harboring the common EGFR L858R point mutation. In contrast, afatinib treatment reduced the proliferation and inhibited the EGFR phosphorylation in L858M/L861Q and L858R mutant cells at similar concentrations. Furthermore, a patient with EGFR L858M/L861Q mutations demonstrated primary resistance to erlotinib and was subsequently treated with afatinib, which resulted in tumor regression (38). Afatinib may therefore be a beneficial therapeutic option for a subset of patients with lung cancer who harbor not only rare EGFR mutations but also compound mutations. Various multiple mutations, including uncommon and compound mutations, might therefore sometimes be detected in clinical practice. The Japan Lung Cancer Society guideline reported the RR of afatinib to be 71%, which is higher than that of gefitinib and erlotinib in actuality (39).
Interestingly, the sensitivity of osimertinib was lower than that of afatinib in cancer cells with compound EGFR mutations (31). As described above, the clinical benefit of osimertinib has been shown to be superior to that of first-generation EGFR-TKIs. However, the survival was not found to differ markedly between patients with L858R mutations treated by the second-generation EGFR-TKI afatinib and those treated by osimertinib. The prevalence of compound EGFR mutations in L858R was relatively high, reaching approximately 20% (31). As a result, T790M mutation clone might remain due to the growth inhibition of afatinib in both uncommon EGFR mutations and compound EGFR mutations. This resulted in the surprising complete and partial RRs of 22% and 88%, respectively, for osimertinib after afatinib treatment (40).
Perspective
First and second-generation TKIs have developed T790M-positive tumors (41,42). Osimertinib has exerted clinical activity and been proven to be effective in a first-line TKI setting (21). However, subsequent treatment options for osimertinib are not clearly defined, and mature OS data are as yet unavailable (21). In the FLAURA trial, the second-generation EGFR-TKI afatinib was not included as a comparator (16). However, long-term survival of Japanese patients of Lux Lung 3 in a post hoc analysis, which reached to 46.9 months is considered to be satisfactory (43). However, these findings must be interpreted with caution, as they were obtained from a relatively small sample (n=54). Nevertheless, the OS in Japanese patients receiving first-line afatinib was extremely encouraging. Furthermore, the median PFS of patients treated by afatinib was 13.8 months, resulting in a post-PFS of 33.1 months (46.9 minus 13.8). New drugs such as ramucirumab,-immune checkpoint inhibitors, and osimertinib might be no administered as a post-progression therapy after afatinib in this study since the data cut-off for the analysis at the time was November 2013, which was before these drugs became available in Japan. The median OS of afatinib followed by osimertinib has not yet been reached (44). Thus, the sequential use of afatinib followed by osimertinib might therefore be a beneficial treatment for patients harboring EGFR mutations.
In contrast, the median PFS of patients treated by osimertinib was reported to be 19.1 months. The resistance mechanisms associated with osimertinib are various and complex. In the future, it is important to consider alternative therapy options, while carefully evaluating local therapy, and patient tolerability for the long-term treatment of such patients (16). Therefore, the optimum first-line choice of treatment remains unclear, and we are looking forward to conducting head-to-head trials of second-generation vs. third-generation EGFR-TKIs as first-line molecular-targeted drugs in patients with EGFR mutations.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pcm.2018.09.07). H Uramoto has received honoraria and research grant from Boehringer Ingelheim. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Torre LA, Bray F, Siegel RL, F, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Uramoto H, Tanaka F. Recurrence after surgery in patients with NSCLC. Transl Lung Cancer Res 2014;3:242-9. [PubMed]
- Uramoto H, Okumura M, Endo S, et al. The 30-day mortality and hospital mortality after chest surgery described in the annual reports published by the Japanese Association for Thoracic and Cardiovascular Surgery. Gen Thorac Cardiovasc Surg 2015;63:279-83. [Crossref] [PubMed]
- Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature 2013;500:415-21. [Crossref] [PubMed]
- Ding L, Getz G, Wheeler DA, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature 2008;455:1069-75. [Crossref] [PubMed]
- Kim MH, Lim SM, Lee K, et al. Can We Prevent Resistance to Osimertinib? Combination or Sequential. J Thorac Oncol 2018;13:877-9. [Crossref] [PubMed]
- Barf T, Kaptein A. Irreversible protein kinase inhibitors: balancing the benefits and risks. J Med Chem 2012;55:6243-62. [Crossref] [PubMed]
- Peters S, Zimmermann S, Adjei AA. Oral epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of non-small cell lung cancer: comparative pharmacokinetics and drug-drug interactions. Cancer Treat Rev 2014;40:917-26. [Crossref] [PubMed]
- Genova C, Rijavec E, Biello F, et al. New systemic strategies for overcoming resistance to targeted therapies in non-small cell lung cancer. Expert Opin Pharmacother 2017;18:19-33. [Crossref] [PubMed]
- Soria JC, Felip E, Cobo M, et al. Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol 2015;16:897-907. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012;489:519-25. [Crossref] [PubMed]
- Ugocsai K, Mandoky L, Tiszlavicz L, et al. Investigation of HER2 overexpression in non-small cell lung cancer. Anticancer Res 2005;25:3061-66. [PubMed]
- Yi ES, Harclerode D, Gondo M, et al. High c-erbB-3 protein expression is associated with shorter survival in advanced non-small cell lung carcinomas. Mod Pathol 1997;10:142-8. [PubMed]
- Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non small-cell lung cancer. N Engl J Med 2015;372:1689-99. [Crossref] [PubMed]
- Girard N. Optimizing outcomes in EGFR mutation-positive NSCLC: which tyrosine kinase inhibitor and when? Future Oncol 2018;14:1117-32. [Crossref] [PubMed]
- Paz-Ares L, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol 2017;28:270-7. [Crossref] [PubMed]
- Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, Phase III trial. Lancet Oncol 2017;18:1454-66. [Crossref] [PubMed]
- Park K, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016;17:577-89. [Crossref] [PubMed]
- Li D, Ambrogio L, Shimamura T, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 2008;27:4702-11. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Yamamoto N, Goto K, Nishio M, et al. Final overall survival in JO22903, a phase II, open-label study of first-line erlotinib for Japanese patients with EGFR mutation-positive non-small-cell lung cancer. Int J Clin Oncol 2017;22:70-8. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Yamamoto G, Kikuchi M, Kobayashi S, et al. Routine genetic testing of lung cancer specimens derived from surgery, bronchoscopy and fluid aspiration by next generation sequencing. Int J Oncol 2017;50:1579-89. [Crossref] [PubMed]
- Uramoto H, Mitsudomi T. Which biomarker predicts benefit from EGFR-TKI treatment for patients with lung cancer? Br J Cancer 2007;96:857-63. [Crossref] [PubMed]
- Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol 2015;16:830-8. [Crossref] [PubMed]
- Kohsaka S, Nagano M, Ueno T, et al. A method of high-throughput functional evaluation of EGFR gene variants of unknown significance in cancer. Sci Transl Med 2017;9:416. [Crossref] [PubMed]
- Watanabe S, Minegishi Y, Yoshizawa H, et al. Effectiveness of gefitinib against non-small-cell lung cancer with the uncommon EGFR mutations G719X and L861Q. J Thorac Oncol 2014;9:189-94. [Crossref] [PubMed]
- Chiu CH, Yang CT, Shih JY, et al. Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Treatment Response in Advanced Lung Adenocarcinomas with G719X/L861Q/S768I Mutations. J Thorac Oncol 2015;10:793-9. [Crossref] [PubMed]
- Katakami N, Atagi S, Goto K, et al. LUX-Lung 4: a phase II trial of afatinib in patients with advanced non-small-cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or both. J Clin Oncol 2013;31:3335-41. [Crossref] [PubMed]
- Yasuda H, Park E, Yun CH, et al. Structural, biochemical, and clinical characterization of epidermal growth factor receptor (EGFR) exon 20 insertion mutations in lung cancer. Sci Translat Med 2013;5:216ra177
- Kim EY, Cho EN, Park HS, et al. Compound EGFR mutation is frequently detected with co-mutations of actionable genes and associated with poor clinical outcome in lung adenocarcinoma. Cancer Biol Ther 2016;17:237-45. [Crossref] [PubMed]
- Watanabe M, Oizumi S, Kiuchi S, et al. The Effectiveness of Afatinib in a Patient with Advanced Lung Adenocarcinoma Harboring Rare G719X and S768I Mutations. Intern Med 2018;57:993-6. [Crossref] [PubMed]
- Saxon JA, Sholl LM, Jänne PA. EGFR L858M/L861Q cis Mutations confer selective sensitivity to afatinib. J Thorac Oncol 2017;12:884-9. [Crossref] [PubMed]
-
NSCLC - Hochmair JM, Schwab S,1 Burghuber O, et al. Prevalence of EGFRT790M mutation in NSCLC patients after afatinib failure, and subsequent response to osimertinib. 18th World Conference on Lung Cancer P2.03-025.
- Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med 2005;2:e73. [Crossref] [PubMed]
- Yang JJ, Zhou Q, Yan HH, et al. A Randomized Controlled Trial of Erlotinib versus Gefitinib in Advanced Non-Small-Cell Lung Cancer Harboring EGFR Mutations (CTONG0901). J Thorac Oncol 2015;10:S321.
- Kato T, Yoshioka H, Okamoto I, et al. Japanese patients with advanced non-small cell lung cancer harboring activating EGFR mutations: Subgroup analysis of LUX-Lung 3. Cancer Sci 2015;106:1202-11. [Crossref] [PubMed]
- Sequist L, Wu Y, Schuler M, et al. Subsequent therapies post-afatinib among patients with EGFR mutation-positive NSCLC in LUX-Lung (LL) 3, 6 and 7. Ann Oncol 2017;28:460-96. [Crossref]
Cite this article as: Uramoto H. What is the best drug as a front-line treatment for EGFR activating mutation? Precis Cancer Med 2018;1:16.

