
Diffuse large B-cell lymphoma with molecular variations more than ABC and GCB classification
Diffuse large B-cell lymphoma (DLBCL), the most common type of lymphoid malignancy in adults, is highly heterogeneous at the clinical and genetic level. The landmark study by Alizadeh et al. almost 20 years ago that used gene expression profiling identified three DLBCL subgroups: activated-B-cell-like (ABC), germinal-center B-cell-like (GCB) and an unclassified group subsequently referred to as the cell-of-origin (COO) classification (1). Biologically, the GCB DLBCLs are derived from B cells of the germinal-center dark zone, where B-cells encounter antigen and somatic hypermutation occurs. In contrast, ABC DLBCLs are liked derived from B cells in the germinal center light zone and/or immediately after B cells leave the germinal center. Pathologic genetic events also differ greatly between these two subgroups, for example, t(14;18) (q32;q21)/IGH-BCL2 is common in GCB tumors whereas constitutive NF-κB activation and enriched genetic aberrations including trisomy 3 and MYD88 mutation are more common in ABC tumors (2,3). As reported in many studies, patients with ABC DLBCL respond less well to standard R-CHOP immunochemotherapy and have a poorer outcome compared to patients with GCB DLBCL (4). However, it need be noted that not all clinical trials have shown that COO classification has prognostic importance (5). COO classification has greatly improved the characterization of DLBCL biology and predicts response to ibrutinib therapy (6). However, approximately 11% of DLBCLs remain unclassified based on the current system and the biological characteristics of these cases are largely undefined (7). Furthermore, within the ABC and GCB subgroups of DLBCL there still remains great heterogeneity in biology and prognosis. For instance, DLBCL cases with MYC and BCL2 translocations (so-called double hit lymphoma) have an extremely poor prognosis, although these neoplasms have a GCB immunophenotype. Additionally, in the ABC subgroup patients with DLBCL that carry MYDL265P and CD79B mutations showed a significantly better response to ibrutinib, a Bruton tyrosine kinase inhibitor, than patients with other ABC DLBCL tumors (6). These data suggest that the current COO classification needs to be refined.
The advent of high-throughput gene sequencing has facilitated a better understanding of the genomic landscape and molecular pathogenesis of DLBCL. Several groups, mostly using whole-exome sequencing, have identified many genetic drivers in DLBCL. Reddy et al. sequenced a total of 1,001 DLBCL cases and used CRISPR screening to explore the biological functions of identified genetic drivers, which contributed to our understanding of the molecular pathogenesis of DLBCL (8). In a recently published paper in the New England Journal of Medicine (9), Schmitz et al. did an analysis of 574 fresh-frozen DLBCL using exome and transcriptome sequencing, deep targeted amplicon resequencing of 372 genes, and DNA copy number analysis. The use of multiple platforms analysis enabled the authors to comprehensively identify genetic events including exonic or splicing site mutations, copy number alterations, and chromosomal rearrangements. An important strength of this study was that the authors enriched for unclassified DLBCL cases (by gene expression profiling), representing 20% of all cases in their study. The authors showed co-occurrence of NOTCH2 mutation and BCL6 fusions in unclassified DLBCL. Additionally, mutations of SPEN, which inhibits NOTCH-dependent gene expression, were found in unclassified DLBCL. The presence of NOTCH2 or SPEN mutations indicates that disruptions affecting the NOTCH pathway constituted crucial genetic events in unclassified DLBCL, providing insights into the molecular pathogenesis of these neoplasms.
The authors further developed an automated algorithm that identified four genetic subtypes of DLBCL: MCD (MYD88L265P and CD79B mutations), BN2 (BCL6 fusion and NOTCH2 mutation), N1 (NOTCH1 mutations) and EZB (EZH2 mutations and BCL2 translocations) (Figure 1). These four subtypes overlapped with classical ABC and GCB subgroups and represented 46.6% of all DLBCL cases. Each of the above subtypes had unique molecular characteristics. MCD cases were exclusively of the ABC subgroup and over 80% of cases had MYD88L265P or CD79B mutations or amplification. MYD88L265P and CD79B abnormalities were both found in 42% of MCD cases and this observation had clinical implications, since DLBCL cases with MYD88L265P and CD79B mutations had a remarkably higher rate of response to ibrutinib (6). Genetic lesions that target HLA-A, HLA-B, HLA-C or CD58, which lead to immune escape, were also highly prevalent in MCD cases. This finding is of interest because DLBCLs arising in immune privileged sites including testis and central nervous system are enriched with MYD88 L265P and CD79B mutations (10,11). These findings suggest that immune surveillance may constitute an important selection pressure for DLBCL with MYD88L265P and CD79B aberrations. The BN2 subtype is characterized by dysregulation of the NOTCH pathway, with 73% cases carrying a NOTCH2 aberrations, SPEN or DTX1 mutations. BCL6 fusion was another BN2 hallmark, which occurred in 73% of all BN2 cases. Coexistence of BCL6 fusion and genetic lesions that disrupt NOTCH signaling (NOTCH2, SPEN, or DTX1 abnormalities) are significantly more frequent in BN2 than in non-BN2 cases, suggesting that these lesions may cooperate in the pathogenesis of the BN2 subtype. Moreover, regulators of NF-κB pathway were frequently disrupted in the BN2 subtype suggesting that NF-κB dysregulation represents another feature of this subset of DLBCLs. The N1 subtype all had NOTCH1 mutations and other acquired aberrations that disrupt B cell differentiation. The EZB cases carried BCL2 translocation or EZH2 mutation as well as aberrations mutations involving tumor suppressor genes involved in epigenetic regulation (CREBBP, EP300, and KMT2D) typical of GCB DLBCL.
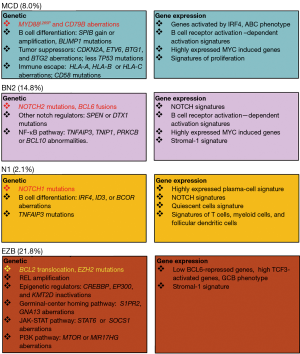
Further, by using RNA-seq data, the authors explored gene expression variances among the different genetic subtypes. Analysis of gene-expression signatures of various signaling or pathways revealed that each genetic subtype has distinct gene expression features. For instance, MCD is characterized by expression of genes that are transcriptionally activated by IRF4, which contributes to the ABC phenotype of MCD. Although both of the N1 and BN2 subtypes showed up-regulated NOTCH signaling, the N1 subtype showed a highly-expressed plasma-cell signature whereas the BN2 subtype displayed enhanced B-cell receptor-dependent NF-κB activation and up-regulation of MYC-induced genes. Regarding tumor microenvironment, gene signatures that represent T cells, myeloid cells, and follicular dendritic cells were remarkably high in the N1 subtype, whereas the BN2 and EZB subtypes showed the previously described “stromal-1” signature, which is related to favorable prognosis in DLBCL treated with immunochemotherapy (12).
Importantly, the four genetic subtypes of DLBCL correlated with different clinical outcomes. Patients with DLBCL of the BN2 and EZB subtypes had significantly longer progression-free survival and overall survival than patients with the MCD and N1 subtypes. Although ABC DLBCLs are considered to have a less favorable outcome, the new genetic subtypes helped to further stratify the ABC subgroup into different risk groups, with the N1 and MCD subtypes of ABC DLBCL displaying a dismal outcome and the BN2 subtype having a relatively favorable prognosis. Similarly, among GCB DLBCL cases, patients with the EZB subtype had a less favorable outcome when compared to patients with other GCB DLCBLs. This result is consistent with the finding that BCL2 translocation predicts worse outcome in GCB DLBCL (13). One might also consider recognizing DLBCL with BCL2 translocation as a separate group.
Another group from the Dana-Farber Cancer Institute also comprehensively explored the genomic landscape of a group of 304 primary DLBCLs (14). In this excellent study which used a different algorithm, designated as non-negative matrix factorization (NMF) consensus clustering, the authors discovered five subsets of DLBCL with distinctive genetic features (C1–C5). The C1 subtype was enriched for BCL6 translocations and carried frequent NOTCH2 or SPEN mutations. The genetic signature of C1 suggests that C1 and BN2 are probably identical. The C3 subtype is mainly composed of GCB DLBCLs and is characterized by BCL2 translocation, with most cases carrying abnormalities affecting epigenetic regulator genes EZH2, CREBBP, or KMT2D. These findings suggest that C3 and EZB cases are very similar. The C5 subtype showed frequent co-occurrence of MYD88L265P and CD79B mutations, a feature that also characterizes the MCD subtype. Clinically, patients with the C3 subtype of GCB DLBCL showed a poorer outcome (versus other GCB tumors) and the C5 subtype of ABCL DLBCL showed a poorer outcome (versus other ABC tumors), similar to the EZB and MCD subtypes in the study by Schmitz et al. Combined, the studies of Schmitz et al. and Chapuy and colleagues suggest that classifications of DLBCL based on coordinate genetic features are reproducible and of clinical importance. However, the N1 subtype, which is characterized by NOTCH1 mutations, was not identified in the Dana-Farber cohort. The explanation for this discrepancy is unknown, but is possibly due to the different studied populations and the low number of N1 cases.
Historically, tumors in general and DLBCL in particular were categorized based on their histologic features and lineage, which, to some extent, could facilitate diagnosis, risk stratification, and treatment decisions. Since the advent of high-throughput profiling of cancer, many efforts have been made to use these methods for tumor subtyping, with some success. Early efforts using gene expression profiling to study DLBCL cases showed three subgroups: ABC, GCB, and unclassified. The study by Schmitz et al. is another step in the process classifying about half of DLCBLs into different genetic groups based on a group of coordinate genetic aberrations rather than individual alterations. Based on this classification, each subtype exhibited distinct genetic, transcriptional as well as clinical characteristics. In the era of precision medicine, genetic systems such as these reported by Schmitz et al. or Chapuy and colleagues will likely be useful in guiding treatment decisions. In the study by Schmitz and colleagues, the MCD subtype will likely respond well to ibrutinib whereas the N1 subtype, which expressed prominent T-cell and plasma cell genes, could possibly benefit from immune-checkpoint blockade. Future studies will likely further refine a genetic classification of DLBCL that will eventually lead to even more precise treatment approaches for patients with DLBCL.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Precision Cancer Medicine. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pcm.2018.06.03). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000;403:503-11. [Crossref] [PubMed]
- Ngo VN, Young RM, Schmitz R, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature 2011;470:115-9. [Crossref] [PubMed]
- Bea S, Zettl A, Wright G, et al. Diffuse large B-cell lymphoma subgroups have distinct genetic profiles that influence tumor biology and improve gene-expression-based survival prediction. Blood 2005;106:3183-90. [Crossref] [PubMed]
- Hu S, Xu-Monette ZY, Tzankov A, et al. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood 2013;121:4021-31; quiz 250. [Crossref] [PubMed]
- Staiger AM, Ziepert M, Horn H, et al. Clinical Impact of the Cell-of-Origin Classification and the MYC/ BCL2 Dual Expresser Status in Diffuse Large B-Cell Lymphoma Treated Within Prospective Clinical Trials of the German High-Grade Non-Hodgkin's Lymphoma Study Group. J Clin Oncol 2017;35:2515-26. [Crossref] [PubMed]
- Wilson WH, Young RM, Schmitz R, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med 2015;21:922-6. [Crossref] [PubMed]
- Scott DW, Mottok A, Ennishi D, et al. Prognostic Significance of Diffuse Large B-Cell Lymphoma Cell of Origin Determined by Digital Gene Expression in Formalin-Fixed Paraffin-Embedded Tissue Biopsies. J Clin Oncol 2015;33:2848-56. [Crossref] [PubMed]
- Reddy A, Zhang J, Davis NS, et al. Genetic and Functional Drivers of Diffuse Large B Cell Lymphoma. Cell 2017;171:481-94.e15. [Crossref] [PubMed]
- Schmitz R, Wright GW, Huang DW, et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N Engl J Med 2018;378:1396-407. [Crossref] [PubMed]
- Nakamura T, Tateishi K, Niwa T, et al. Recurrent mutations of CD79B and MYD88 are the hallmark of primary central nervous system lymphomas. Neuropathol Appl Neurobiol 2016;42:279-90. [Crossref] [PubMed]
- Kraan W, Horlings HM, van Keimpema M, et al. High prevalence of oncogenic MYD88 and CD79B mutations in diffuse large B-cell lymphomas presenting at immune-privileged sites. Blood Cancer J 2013;3:e139. [Crossref] [PubMed]
- Lenz G, Wright G, Dave SS, et al. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med 2008;359:2313-23. [Crossref] [PubMed]
- Visco C, Tzankov A, Xu-Monette ZY, et al. Patients with diffuse large B-cell lymphoma of germinal center origin with BCL2 translocations have poor outcome, irrespective of MYC status: a report from an International DLBCL rituximab-CHOP Consortium Program Study. Haematologica 2013;98:255-63. [Crossref] [PubMed]
- Chapuy B, Stewart C, Dunford AJ, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med 2018;24:679-90. [Crossref] [PubMed]
Cite this article as: Miao Y, Medeiros LJ, Li J, Young KH. Diffuse large B-cell lymphoma with molecular variations more than ABC and GCB classification. Precis Cancer Med 2018;1:4.

